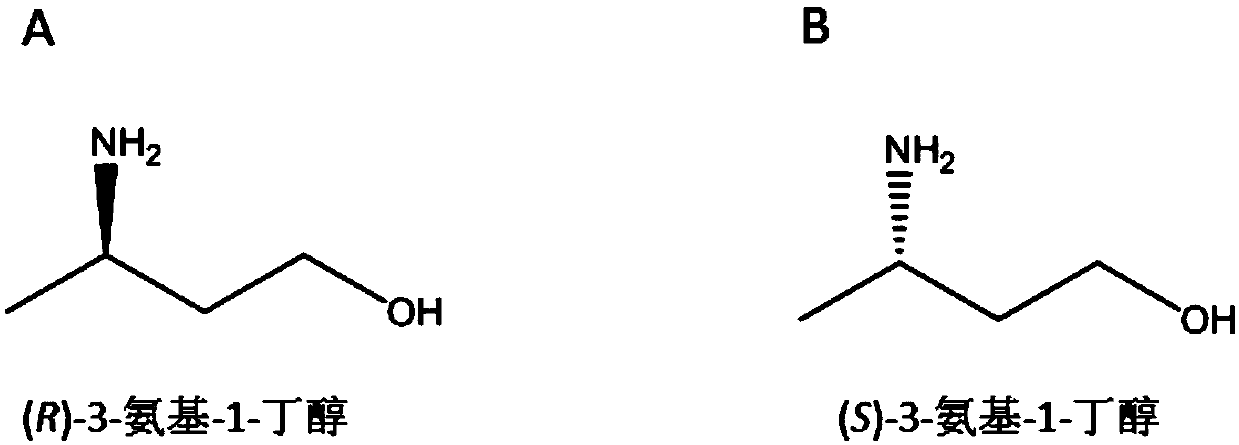
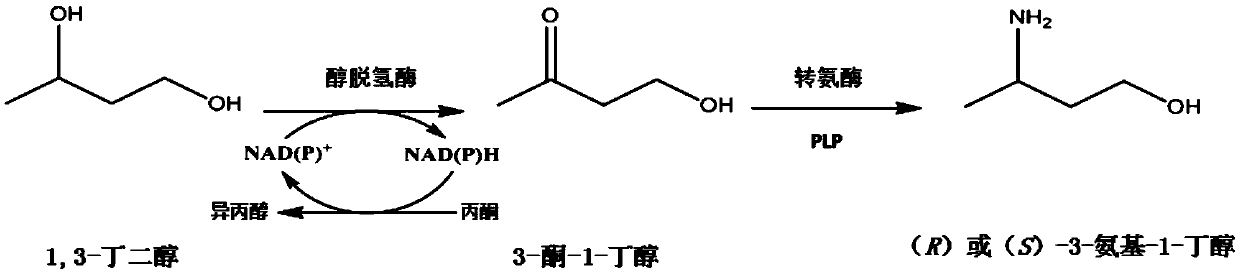
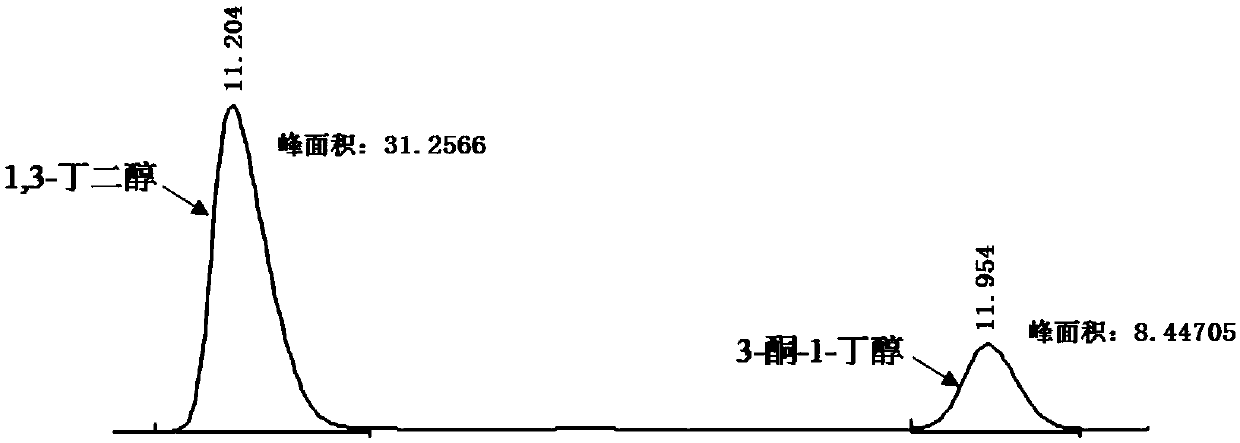
Synthetic method of chiral 3-amino-1-butanol
A synthetic method, amino technology, applied in botany equipment and methods, biochemical equipment and methods, genetic engineering, etc., can solve problems such as difficult industrialization and lengthy synthetic routes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0120] Example 1. Alcohol dehydrogenase coupled transaminase to prepare chiral 3-amino-1-butanol
[0121] One, the preparation of engineering bacteria of alcohol dehydrogenase or its mutant, transaminase
[0122] The genes encoding the relevant enzymes are synthesized separately (codon optimization is performed with Escherichia coli as the host if necessary), and the synthesized genes are connected to various expression vectors to construct. The expression vectors are various conventional vectors in the art. The vector of the present invention is specifically pET22b(+), after inserting the coding gene of the relevant enzyme after the whole gene synthesis into the restriction site NdeI and XhoI of pET22b(+), and after the sequence verification is correct, the recombinant vector is obtained . The related gene mutants were obtained by site-directed mutagenesis.
[0123] The correct recombinant expression vector verified by the above sequencing was transformed into a suitable m...
Embodiment 2
[0153] Example 2, Co-expression of Enzyme A and Enzyme B Whole Cell Preparation of Chiral 3-amino-1-butanol
[0154] Enzyme A is an alcohol dehydrogenase shown in Table 1 or a mutant thereof. Enzyme B is the transaminase shown in Table 1.
[0155] 1. Preparation of engineering bacteria co-expressing enzyme A and enzyme B
[0156] The genes encoding the relevant enzymes are synthesized separately (codon optimization is performed with Escherichia coli as the host if necessary), and the synthesized genes are connected to various expression vectors to construct. The expression vectors are various conventional vectors in the art. The vector of the present invention is specifically pETDuet-1, the DNA fragment of enzyme A after the whole gene synthesis is inserted between the restriction sites EcoRI and HindIII of pETDuet-1, and the DNA fragment of enzyme B after the whole gene synthesis is inserted into pETDuet- 1 between NdeI and XhoI. The recombinant vector was transformed int...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com