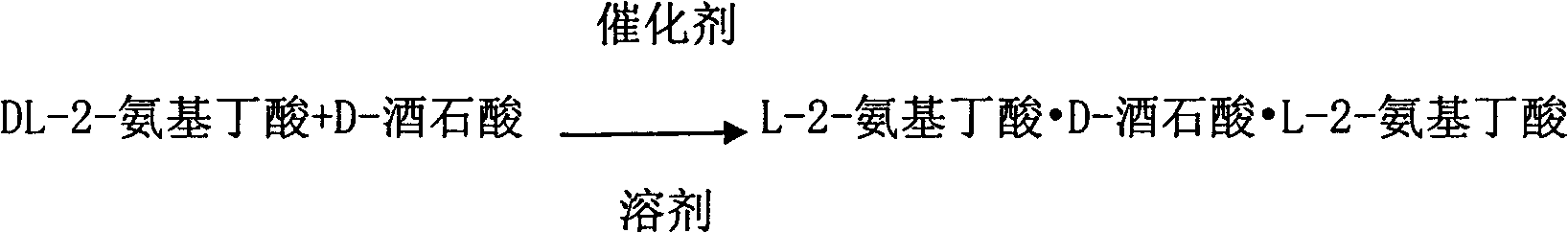
Method for preparing L-2-aminobutyric acid by asymmetric conversion method
A technology of aminobutyric acid and DL-2-, which is applied in the field of biochemistry to achieve the effects of reducing production costs, high yields, and reducing usage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
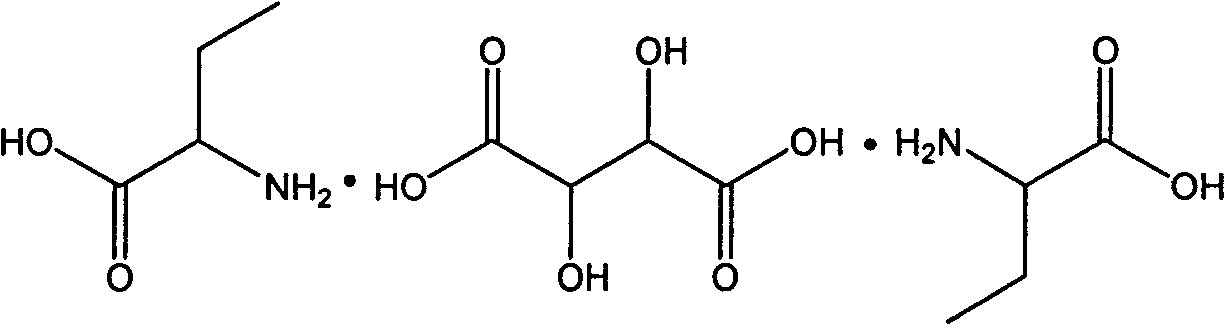
[0025] Add a mixture of 20.6g (0.2mol) DL-2-aminobutyric acid and 15.1g (0.1mol) D-tartaric acid to 375ml of propionic acid, add 0.7g of benzaldehyde, react at 100°C for 6 hours, and cool to room temperature Immediately afterwards, cool with an ice-water bath for 0.5 hours, filter, and the mother liquor can be recycled, and the solid obtained by filtering is dissolved in water, and recrystallized to obtain 32.3 g of L-2-aminobutyric acid D-tartaric acid L-2-aminobutyrate, as 90.5% of theoretical yield.
Embodiment 2
[0027] Add a mixture of 30.9g (0.3mol) DL-2-aminobutyric acid and 15.1g (0.1mol) D-tartaric acid into 400ml of dilute hydrochloric acid, add 2.2g of methylbenzaldehyde, and react at 90°C for 8 hours. After cooling to room temperature, cool in an ice-water bath for 0.5 hour, filter, and the mother liquor can be recycled. The solid was dissolved in water and recrystallized to obtain 34.2 g of L-2-aminobutyric acid·D-tartaric acid·L-2-aminobutyric acid salt, which was 95.8% of the theoretical yield.
Embodiment 3
[0029] Add a mixture of 20.6g (0.2mol) DL-2-aminobutyric acid and 15.1g (0.1mol) D-tartaric acid into 375ml of acetic acid, add 0.7g of 2-hydroxynaphthaldehyde, react at 70°C for 6 hours, and cool After reaching room temperature, cool in an ice-water bath for 0.5 hour, filter, and the mother liquor can be recycled. The solid was dissolved in water and recrystallized to obtain 32.1 g of L-2-aminobutyric acid·D-tartaric acid·L-2-aminobutyric acid salt, which was 89.9% of the theoretical yield.
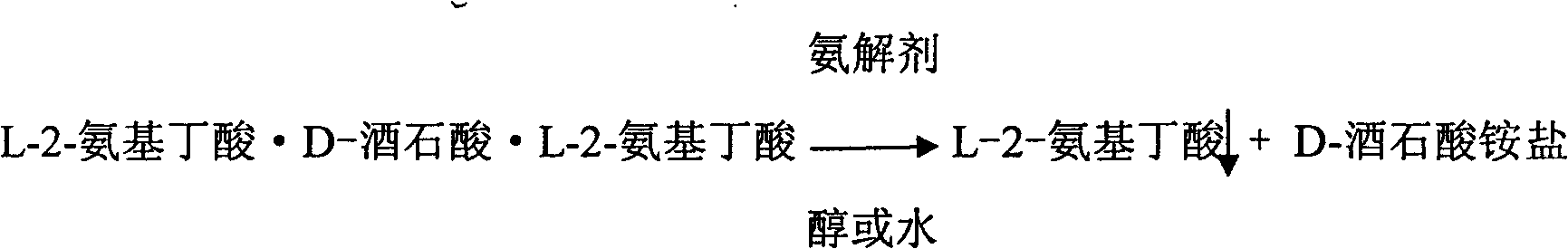
[0030] Preparation of L-2-aminobutyric acid from L-2-aminobutyric acid·D-tartaric acid·L-2-aminobutyric acid salt
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com