Threonine deaminase mutant as well as preparation method and application thereof
A threonine deaminase and mutant technology, which is applied in the directions of botanical equipment and methods, biochemical equipment and methods, and applications, can solve problems such as poor stability, increase costs, limit the service life of biocatalysts, etc. Industrialization, the effect of improving thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1: Construction of threonine deaminase recombinant bacteria
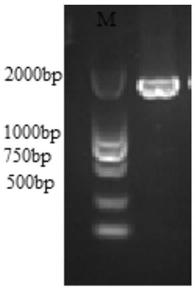
[0021] Threonine deaminase derived from Escherichia coli K12 was selected, using PCR technology, using the E.coli K12 genome as a template, ilvA-F: 5'-GGAATTCCATATGGCTGACTCGCAACCCCT G-3' and ilvA-R: 5'-CCGCTCGAGTTAACCCGCCAAAAAGAAC-3 ' as a primer to amplify threonine deaminase, and introduce Nde I and Xho I restriction enzyme sites at its 5' end and 3' end, respectively. The PCR reaction system (50 μL) was: 10 μL of 5×PrimeSTAR GXL buffer, 1 μL of PrimeSTAR GXL DNA Polymerase, 4 μL of 2.5mM dNTPs, 1 μL of template DNA, 1 μL of upstream and downstream primers, and 32 μL of sterile water. The PCR reaction conditions were pre-denaturation at 94°C for 5 min; denaturation at 94°C for 30 s, annealing at 55°C for 30 s, extension at 68°C for 2 min, and 30 cycles; extension at 68°C for 10 min. Verify and recover the PCR amplification product with 1% agarose gel electrophoresis, the result is amplified to t...
Embodiment 2
[0023] Embodiment 2: Directed evolution mutation of threonine deaminase gene
[0024] Extract the recombinant Escherichia coli BL21(DE3) / pET28a(+)-ilvA plasmid in Example 1, use the plasmid pET28a(+)-ilvA as a template, and use Primers-F: 5'-CGCGGATCCATGGCTGACTCG A-3', Primers- R: 5'-CCCAAGCTTAACCCGCCAAAAAGAAC-3' is used as a primer to introduce replication errors into the polymerase chain reaction by adding divalent manganese ions and divalent magnesium ions. The PCR reaction system for directed evolution is: 5mM MnCl 2 1 μL, Taq polymerase 0.5 μL, 10× buffer 10 μL, 25mM MgCl 2 14 μL, 4 μL of dNTP Mixture, 1 μL of upper and lower primers, 0.5 μL of template DNA (50ng / μL) and 18 μL of sterile water. PCR reaction conditions for directed evolution: 94°C for 5min; 30 cycles of 94°C for 30s, 55°C for 30s, 72°C for 3min; 72°C for 10min.
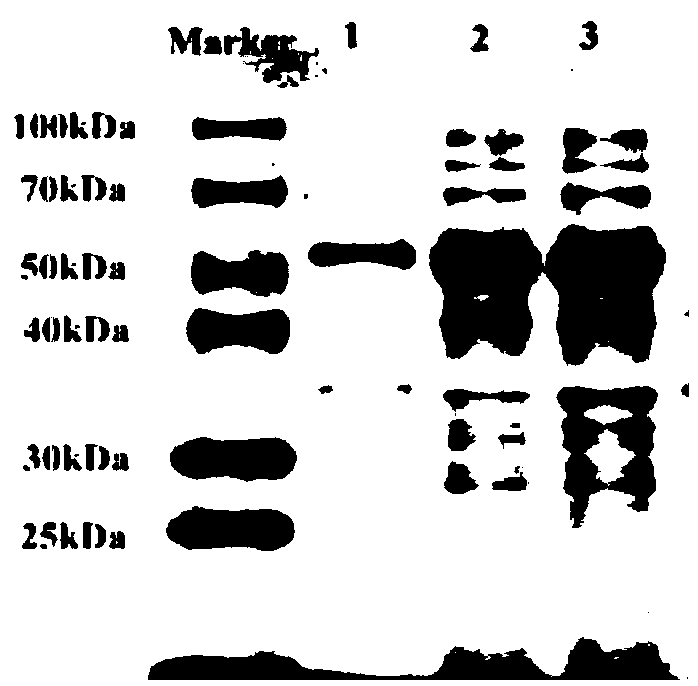
[0025] The PCR products were purified and treated with restriction endonucleases BamH I and Hind III at 37°C for 3 hours. The PCR product a...
Embodiment 3
[0028] Example 3: Escherichia coli K12 Threonine Deaminase Gene Multiple Sequence Alignment and Computer Simulation Mutation
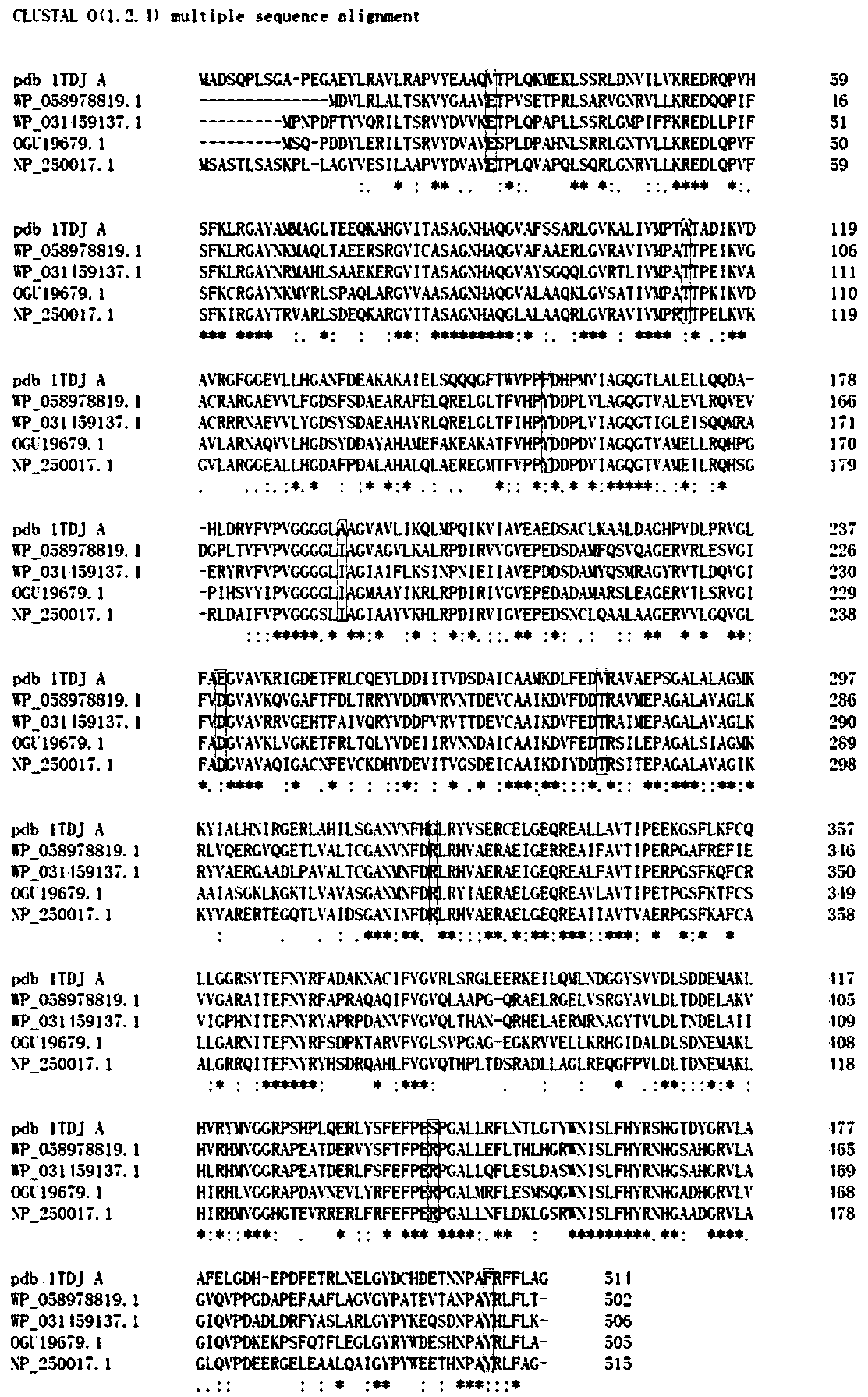
[0029] Through BLAST search, it was found that threonine deaminase belongs to Trp-synth-beta II superfamily (accession ID: cd01562). And found four different strains of thermostable threonine deaminase. They are Deinococcus-Thermus (accession ID: WP_058978819.1), Chloroflexus sp.MS-G (accession ID: WP_031459137.1), Hydrogenophilales bacterium (accession ID: OGU19679.1) and Pseudomonas aeruginosa (accession ID: NP_1500 ). According to the results of amino acid sequence alignment (such as image 3 ), and found 9 possible amino acid residues, namely: Val131, Ala114, Phe157, Ala193, Glu240, Val281, Gly323, Ser443, Phe510. The "weighted mutation energy" of the mutant strains Val131Phe, Ala114Pro, Phe157Tyr, Ala193Ile, Glu240Asp, Val281Thr, Gly323Arg, Ser443Arg, and Phe510Leu was calculated by computer simulation mutation, and the mutation type with the s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com