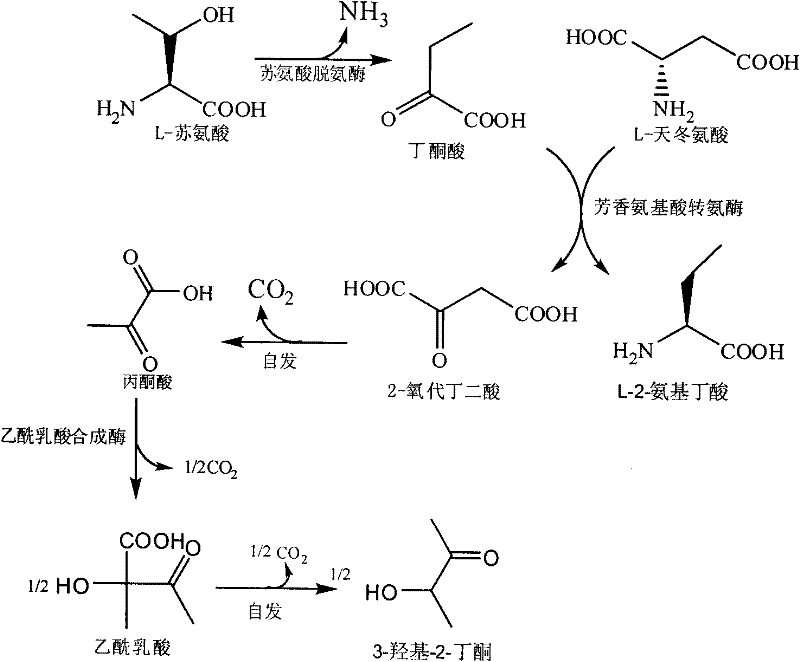
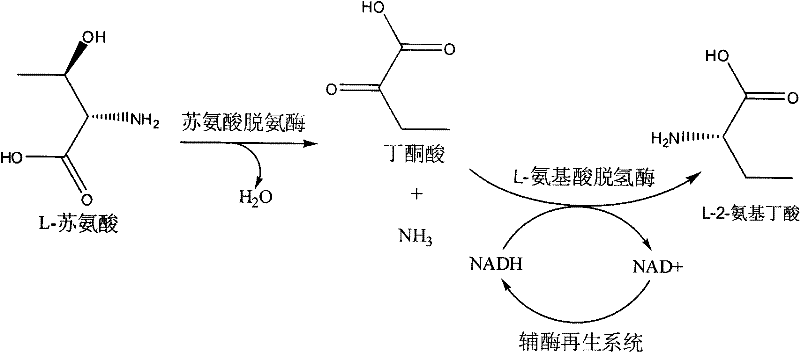
Method for producing L-2-aminobutyric acid
A production method, the technology of aminobutyric acid, applied in the biological field, can solve the problems of high cost, unstable properties of butyronic acid, and restrictions on industrial application, and achieve the effects of low process cost, high conversion rate and product concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1 The reaction system of threonine deaminase derived from Escherichia coli, leucine dehydrogenase derived from Bacillus cereus and glucose dehydrogenase derived from Bacillus subtilis catalyzes the production of L-2-aminobutyric acid
[0026] Prepare liquid medium TB (pH7.0-7.5), containing 12g / L peptone, 24g / L imported yeast extract, 5g / L glycerol, 2.13g / L KH 2 PO 4 , 16.43g / L K 2 HPO 4 ·3H 2 O. Medium TB was divided into 1L Erlenmeyer shake flasks with a volume of 200mL, and then heated and sterilized in an autoclave at 121°C for 20min.
[0027] Leucine dehydrogenase expression strains derived from Bacillus cereus (refer to Kula MR, Stoyan T, Recktenwald A. Cloning, sequencing and overexpression of the leucine dehydrogenasegene from Bacillus cereus. Journal of Biotechnology. 1997, 54: 77 -80 described method construction), Bacillus subtilis derived glucose dehydrogenase expression bacterial species (with reference to Lampel KA, et al. 43) and Escherichia...
Embodiment 2
[0033] Example 2 The reaction system of threonine deaminase derived from Escherichia coli, leucine dehydrogenase derived from Bacillus cereus and formate dehydrogenase derived from Candida boidinii catalyzes the production of L-2-aminobutyric acid
[0034] The strain fermentation, the preparation of the crude enzyme solution and the liquid phase detection method are the same as in Example 1, wherein, the formate dehydrogenase expression strain derived from Candida boidinii refers to Sakai Y, et al. Regulation of the formatedehydrogenase gene, FDH1 , in the methylotrophic yeast Candida boidinii and growth characteristics of an FDH1-disrupted strain on methanol, methylamine, and choline. JBacteriol.1997, 179 (14): 4480-5 described method construction.
[0035] The enzyme activity detection method of threonine deaminase derived from Escherichia coli and leucine dehydrogenase derived from Bacillus cereus is the same as that in Example 1.
[0036] The detection method of formate de...
Embodiment 3
[0038] Example 3 Valine dehydrogenase derived from Streptomyces albicans, threonine deaminase derived from Escherichia coli and glucose dehydrogenase derived from Bacillus subtilis catalyzed by the reaction system to produce L-2-aminobutyric acid
[0039] Strain fermentation, preparation of crude enzyme solution and liquid phase detection method are the same as in Example 1, wherein, the expression strain of valine dehydrogenase derived from Streptomyces albicans refers to Hyuu CG, Kim SS et al.Valine dehydrogenase from Streptomyces albus gene cloning heterologous expression and identification of active site by site-directed mutagenesis. FEMS Microbiology Letters. 2000, 182: 29-34 described method construction.
[0040] The enzyme activity detection method of threonine deaminase derived from Escherichia coli and glucose dehydrogenase derived from Bacillus subtilis is the same as that in Example 1.
[0041] The detection method of valine dehydrogenase enzyme activity derived fr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com