Recombination co-expression system for preparing L-2-aminobutyric acid and application thereof
A co-expression technology of aminobutyric acid, applied in the field of genetic engineering, can solve the problems of limited industrial application and high production cost, and achieve the effects of strong cell tolerance, high production efficiency and accelerated reaction speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
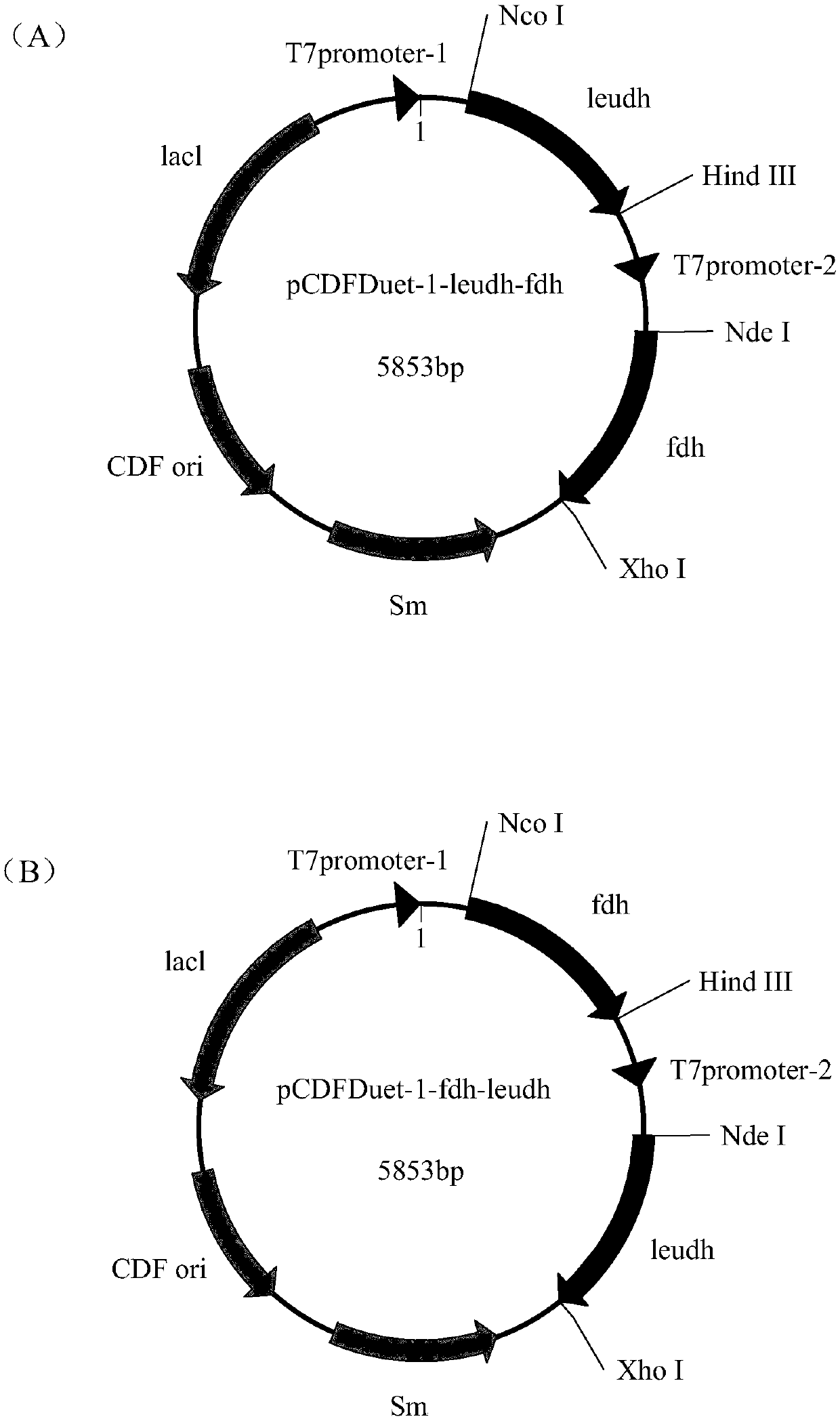
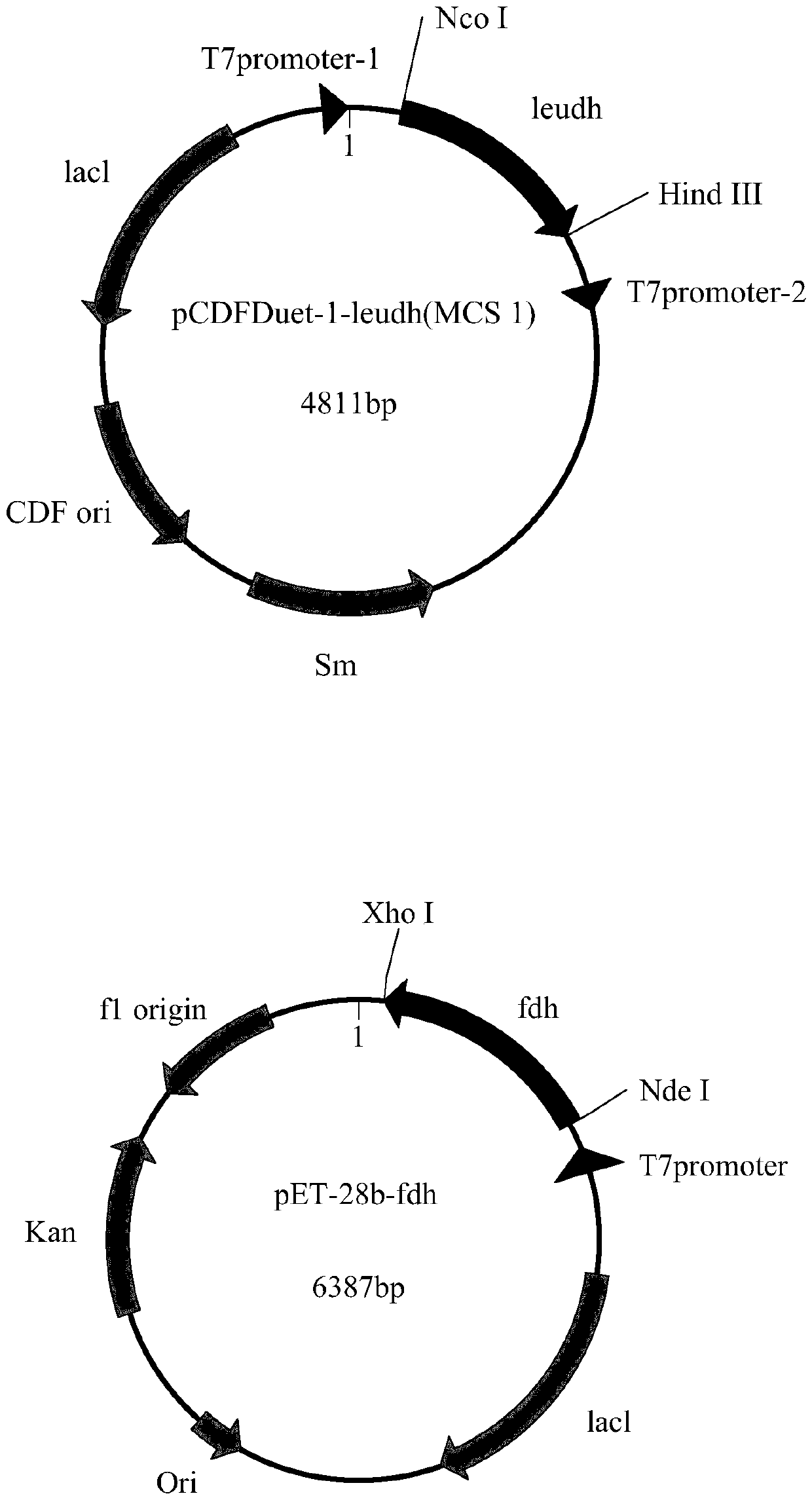
[0042] Example 1: Leucine dehydrogenase gene (LeuDH) and formate dehydrogenase gene (FDH) cloning and co-expression recombinant system construction
[0043] The gene of leucine dehydrogenase is derived from the whole genome sequence of Thermoactinomyces intermedius. In order to express the protein with His-tag after the gene is connected to the vector pET-28b, its stop codon was excised, and its sequence and common restriction endonuclease were compared with the codon preference of B. subtilis 168 as a reference Recognition sites BamH I, Xho I, Pst I, Hind III and Nco I were sequence optimized to obtain a new leucine dehydrogenase gene (the nucleotide sequence is SEQ ID NO.1, and the amino acid sequence is SEQ ID NO. 2) It has been disclosed in the patent application (201610867380.8), and the new leucine dehydrogenase gene has been connected to the expression vector pET-28b, namely pET-28b-leudh.
[0044] The formate dehydrogenase gene (nucleotide sequence is SEQ ID NO.3, ami...
Embodiment 2
[0056] Example 2: Expression of leucine dehydrogenase and formate dehydrogenase in different co-expression systems
[0057] The different co-expression strains constructed in Example 1 above were inoculated into shake flasks containing 50 mL of LB liquid medium, and corresponding resistance was added, and cultured on a shaker at 37°C and 150 r / min for 12 hours to obtain seed liquid. The seed solution was transferred to 100mL LB liquid medium with an inoculum volume fraction of 1% (v / v), and resistance was added, and cultured to OD at 37°C and 180r / min 600 When it reaches 0.6-0.8, add the inducer IPTG to the final concentration of 0.1mmol / L, the induction temperature is 22°C, and the induction time is 14h. After fermentation, centrifuge at 4°C, 8000r / min, 10min, discard the supernatant, wash twice with normal saline, collect the cells, and store at 4°C for later use.
Embodiment 3
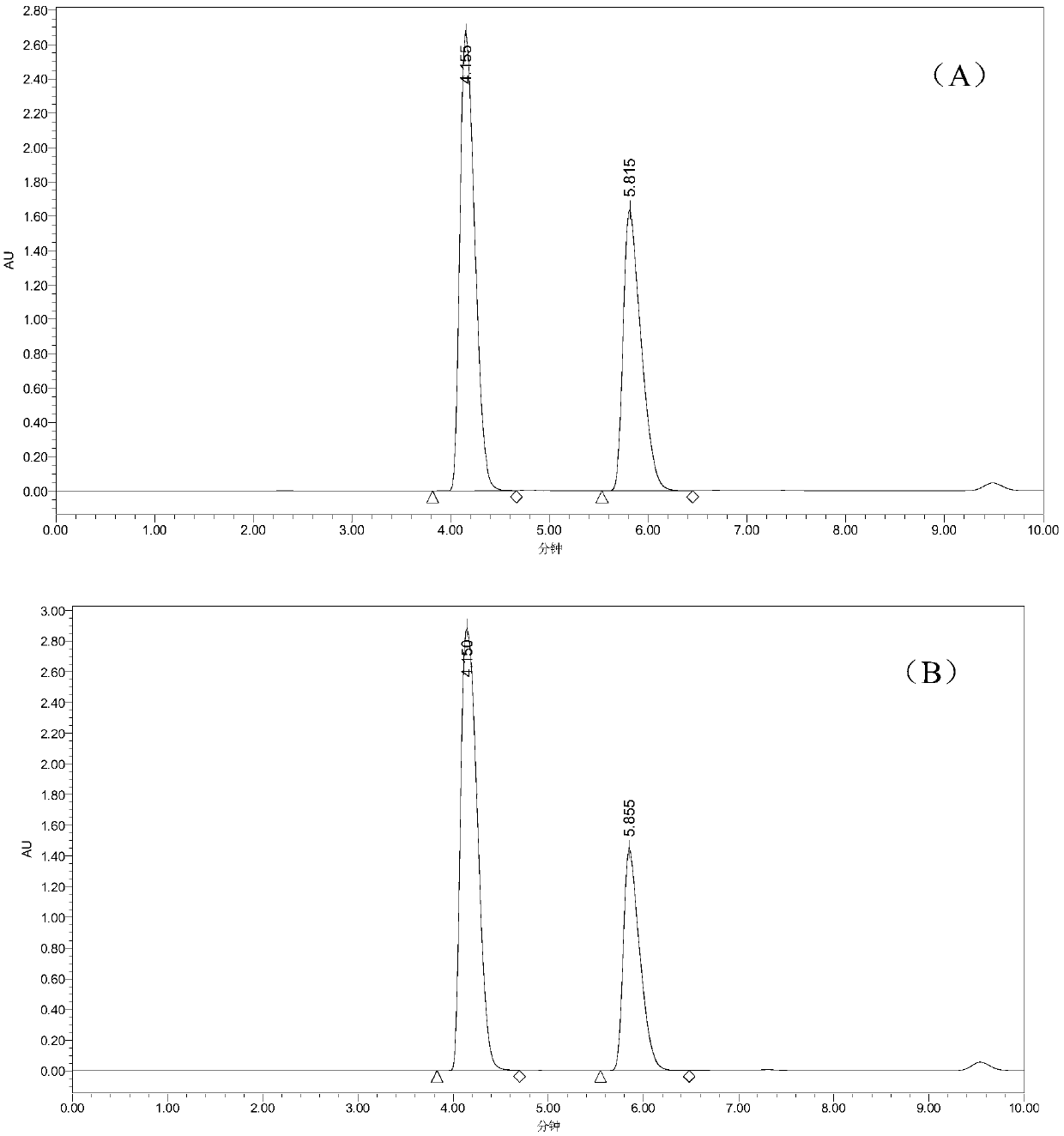
[0058] Example 3: Comparison of the efficiency and specific enzyme activity of different co-expression strains producing L-2-aminobutyric acid
[0059] Definition of enzyme activity: Under standard conditions, the amount of enzyme required to generate 1 μmol L-2-aminobutyric acid per minute is 1 unit of enzyme activity, and the apparent specific enzyme activity is the number of enzyme activity units per gram of wet bacteria .
[0060] Sample detection method: American waters high performance liquid chromatography, chromatographic column Eclipse XD8-C18 (5 μm 4.6mm × 250mm), mobile phase: 0.02mol / L disodium hydrogen phosphate (pH 7.2): acetonitrile=70:30, flow rate : 1.0mL / min, column temperature: 30°C, UV detection wavelength: 360nm.
[0061] Sample derivatization conditions: take 100 μL of the sample to be tested, and 100 μL of 0.5mol / L NaHCO 3 The solution was mixed with 50 μL of 1% (v / v) 2,4-dinitrofluorophenylacetonitrile solution, kept at 60°C in the dark for 1 hour, co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com