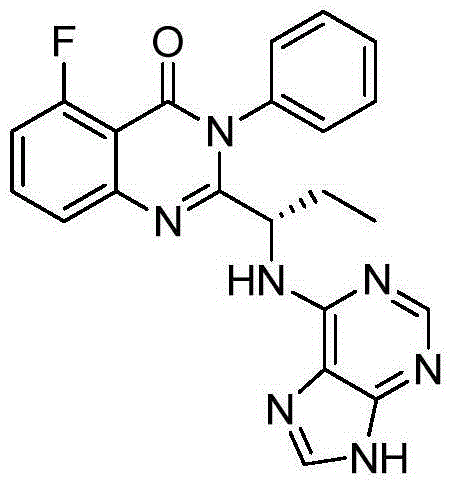
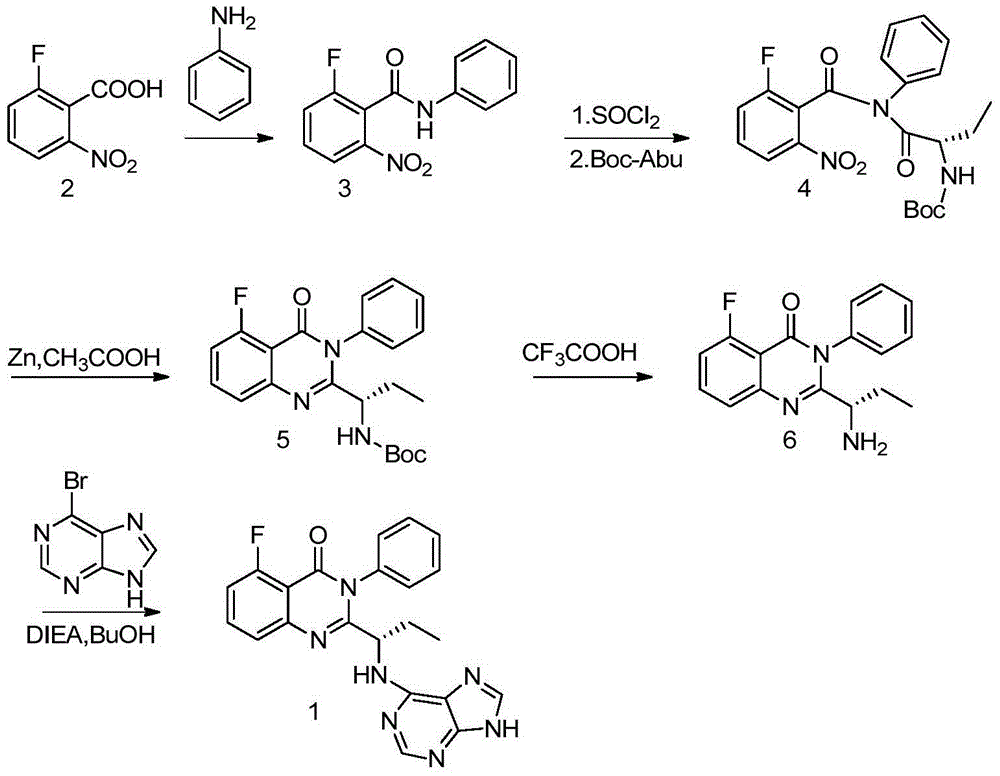
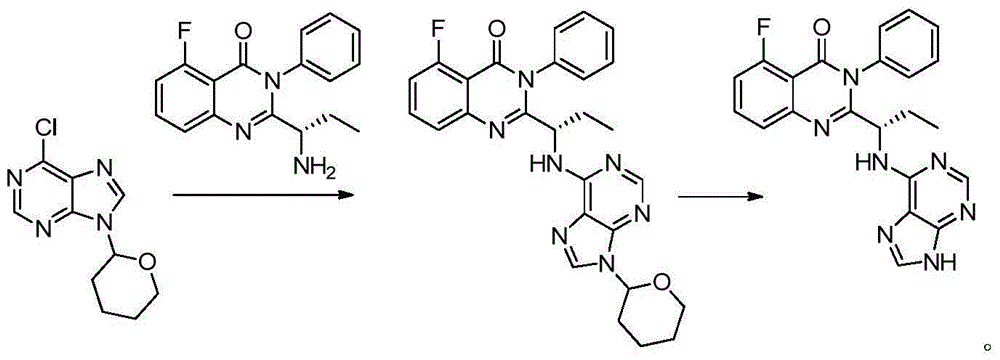
Idelalisib preparation method
A phenyl and purine technology, applied in the field of Idelalisib preparation, can solve the problems of only 50% yield and expensive 6-bromopurine, etc., and achieve the effect of simple post-treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-2
[0015] Example 1-2: Preparation of 6-chloro-9-(tetrahydro-2-pyranyl)-purine
[0016] Dissolve 6-chloropurine (15.5g) and p-toluenesulfonic acid (0.26g) in ethyl acetate (180ml), heat to 50°C, and slowly drop 2,3 dihydropyran (10.5ml) into the reaction After dripping, continue to stir at this temperature for 1 hour, slowly cool to room temperature, add saturated ammonium chloride solution (10ml) under stirring, then wash twice with water and once with salt water, dry, concentrate to obtain an oily crude product, and use n-hexane Recrystallization of alkane (150ml) gave 19.6g of 6-chloro-9-(tetrahydro-2-pyranyl)-purine, yield 82.1%. 1 H NMR (400MHz,d6-DMSO)δ8.91(s,1H),8.82(s,1H),5.80(d,1H),4.04(m,1H),3.75(m,1H),2.35(m, 1H),2.01(m,2H),1.76(m,1H),1.62(m,2H).ESI-MS(m / z):239[M+H] +
[0017] Dissolve 6-chloropurine (15.5g) and p-toluenesulfonic acid (0.26g) in ethyl acetate (180ml), heat to 50°C, and slowly drop 2,3 dihydropyran (10.5ml) into the reaction After dripping, continue...
Embodiment 3-4
[0018] Example 3-4: (S)-5-fluoro-3-phenyl-2-{1-[(9-tetrahydro-2H-pyran-2-yl)-9H-purin-6-ylamino] Preparation of propyl}-3H-quinazolin-4-one
[0019] 6-Chloro-9-(tetrahydro-2-pyranyl)-purine (14.31g), (S)-2-(1-amino-propyl)-5-fluoro-3-phenyl-3H- Quinazolin-4-one (8.91g) and triethylamine (9.54g) were dissolved in isopropanol (40ml), refluxed at 80°C for 24 hours, cooled at room temperature, filtered, washed with isopropanol, washed with water, n-hexane The filter cake was washed to give (S)-5-fluoro-3-phenyl-2-{1-[(9-tetrahydro-2H-pyran-2-yl)-9H-purin-6-ylamino]propane Base}-3H-quinazolin-4-one 12.8g, yield 85.3%.
[0020] 1 H NMR (400MHz, CDCl 3 )δ12.01(s,1H),8.28(s,1H),8.02(s,1H),7.71–7.63(m,1H),7.63–7.45(m,5H),7.35(d,J=7.4Hz ,1H),7.15–7.05(m,1H),5.71(d,J=10.1Hz,1H),4.21–4.12(m,1H),3.78(t,J=11.3Hz,1H),3.12(qd, J=7.3,4.9Hz,6H), 2.07–1.79(m,2H),0.87(t,J=7.4Hz,3H).ESI-MS(m / z):500[M+H] +
[0021] 6-Chloro-9-(tetrahydro-2-pyranyl)-purine (14.31g), (S)-2-(1-amino-propyl)-5-...
Embodiment 5-6
[0022] Embodiment 5-6: Preparation of Idelalisib
[0023] Treat (S)-5-fluoro-3-phenyl-2-{1-[(9-tetrahydro-2H-pyran-2-yl)-9H-purin-6-ylamino with hydrochloric acid ethanol (40ml) ]Propyl}-3H-quinazolin-4-one (10g), stirred at room temperature for 3 hours, adjusted the pH to about 8-9 with ammonia water, added seed crystals, and filtered to obtain (S)-2-(1-amino- Propyl)-5-fluoro-3-phenyl-3H-quinazolin-4-one 7.3 g, yield 87.9%.
[0024] 1 H NMR (400MHz, DMSO) δ10.46(s, 1H), 10.11(s, 1H), 8.14(d, J=17.4Hz, 2H), 7.87(d, J=8.1Hz, 1H), 7.77(s ,1H),7.54–7.39(m,3H),7.27(t,J=7.7Hz,2H),7.09(t,J=7.4Hz,2H),4.72(s,1H),2.07–1.79(m, 2H), 0.94(t, J=7.3Hz, 3H).ESI-MS(m / z): 416[M+H] +
[0025] (S)-5-fluoro-3-phenyl-2-{1-[(9-tetrahydro-2H-pyran-2-yl)-9H-purin-6-ylamino]propyl}-3H -Quinazolin-4-one (10g) was dissolved in hydrochloric acid ethanol (40ml), stirred at room temperature for 3 hours, washed with saturated NaHCO 3 The solution was quenched, and the pH was adjusted to about 7-8, e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com