A kind of idelalisib crystal form a and preparation method thereof
A crystal form and solution technology, applied in the field of medicinal chemistry, can solve problems such as unfavorable absorption, poor solubility, poor solubility, etc., and achieve the effects of good solubility, short production cycle, and stable quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Embodiment 1: Preparation of Idelalisib Form A
Embodiment 11
[0061] At 30°C, dissolve 1g of Idelalisib raw material (regardless of its form) in 30mL of acetone to obtain a clear solution; at 30°C, slowly add anti-solvent water to the above solution until initial turbidity (about 100mL) ; Insulated and stirred to crystallize for 2 hours, cooled to room temperature; filtered and dried to obtain 0.85 g of crystals; the yield was 85%, and the HPLC purity was 99.58%.
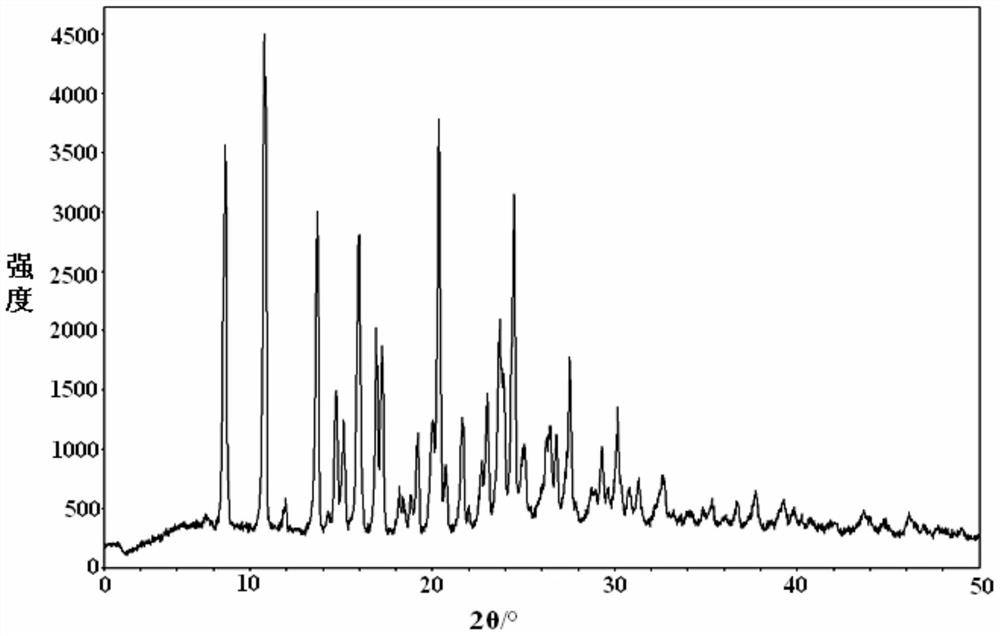
[0062] figure 1 is the XRD spectrum of the obtained crystal, by figure 1 It can be seen that under powder X-ray diffraction, the crystal has Characteristic peaks with relative intensities greater than 50%; 8.5±0.2°, 10.7±0.2°, 13.6±0.2°, 14.6±0.2°, 15.0±0.2°, 15.8±0.2°, 16.8±0.2°, 17.1±0.2° in 2θ °, 19.9±0.2°, 20.2±0.2°, 22.9±0.2°, 23.6±0.2°, 23.7±0.2°, 24.3±0.2°, 26.3±0.2° have characteristic peaks with relative intensities greater than 30%.
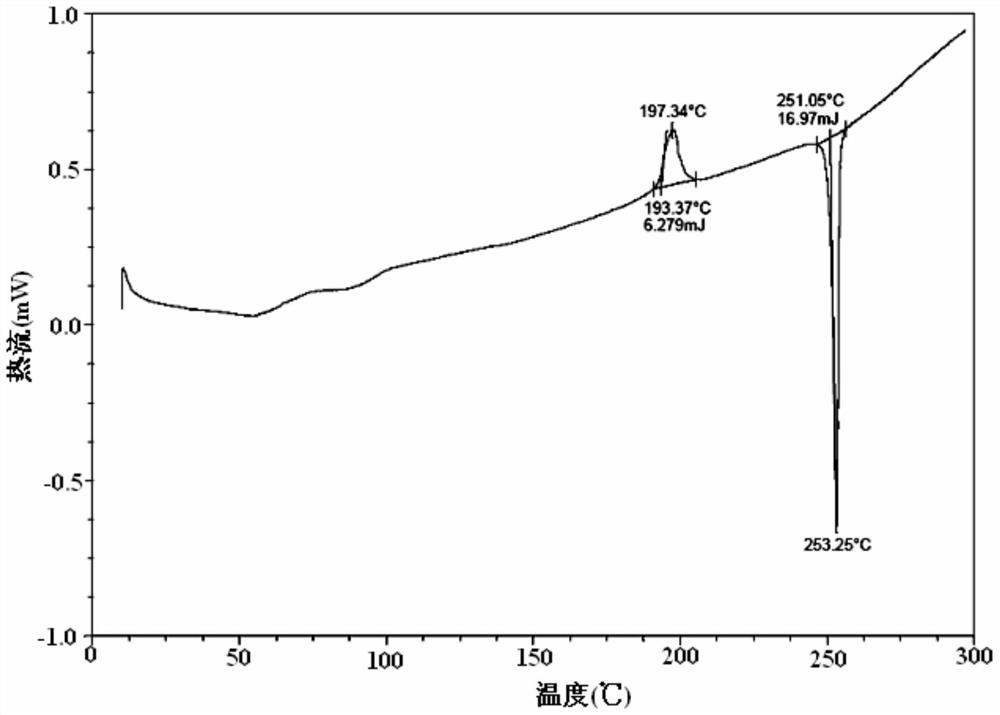
[0063] figure 2 For the DSC spectrum of the obtained crystal, by figure 2 It can be seen that the crystal has a solvent endot...
Embodiment 12
[0067] At 65°C, dissolve 1g of Idelalisib raw material (regardless of its form) in 20mL of acetonitrile to obtain a clear solution; at 50°C, slowly add anti-solvent water to the above solution until initial turbidity (about 110mL) ; Insulated and stirred to crystallize for 2 hours, cooled to room temperature; filtered and dried to obtain 0.90 g of crystals; the yield was 90%, and the HPLC purity was 99.70%.
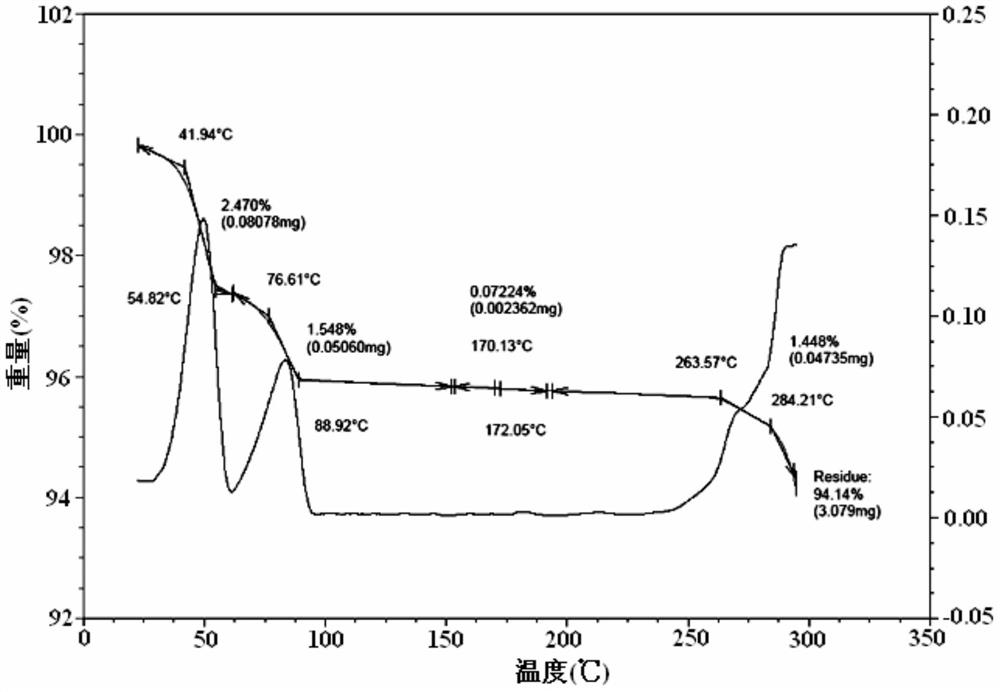
[0068] After determination and analysis, the obtained crystals have figure 1 Shown XRD spectrogram feature and figure 2 The DSC spectral features shown and image 3 The characteristics of the TGA spectrum shown in this example illustrate that the crystal obtained in this example is also Idelalisib crystal form A of the present invention.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com