Idelalisib crystal form C and preparation method thereof
A technology of Idelalis and its crystal form, which is applied in the field of medicinal chemistry and can solve problems such as poor solubility, large static electricity, and crystal form A with large static electricity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0100] In a preferred embodiment of the present invention, the preparation method comprises the following steps:
[0101] a) Dissolving the ederalix raw material in an organic solvent, the dissolving temperature is 15-120°C (preferably 20°C-80°C) to obtain a clear solution;
[0102] b) Concentrate the above solution at 20-40°C until a small amount of turbidity occurs;
[0103] c) at 0~60°C (preferably 0~50°C), dropwise add anti-solvent water to the solution obtained in step a) for crystallization;
[0104] d) heat preservation and stirring;
[0105] e) cooling to room temperature, filtering and drying to obtain the idelalix crystal form C.
[0106] The crystal form of the idelalix raw material is not limited, and it can be amorphous or any known crystal form or a mixture thereof.
[0107] Said solvent refers to the solvent that can completely dissolve the raw material of ederalix in it at 15~120℃, including but not limited to methanol, ethanol, acetone, acetonitrile, tetrah...
Embodiment 1
[0130] Example 1: Preparation of idelalix crystal form C
Embodiment 11
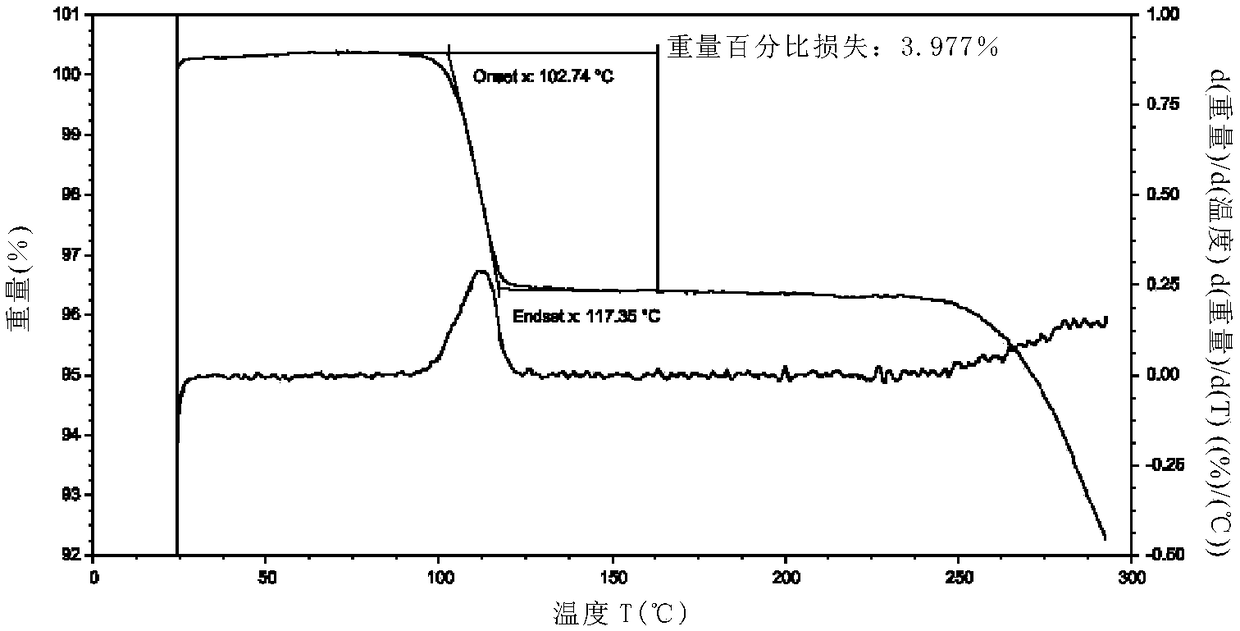
[0132] At 40°C, 1 g of ederalix raw material (crystal form I referred to in the WO2015014315 international patent application) was dissolved in 30 mL of tetrahydrofuran to obtain a clear solution; the solution was concentrated at 25°C until turbidity appeared; At 40°C, anti-solvent water was slowly added dropwise to the above solution, and after the solid was precipitated, the crystallization was carried out for 2 hours under heat preservation and stirring, and then cooled to room temperature; filtered and dried to obtain 0.90 g of crystal; the yield was 90%, and the HPLC purity was 99.2% .
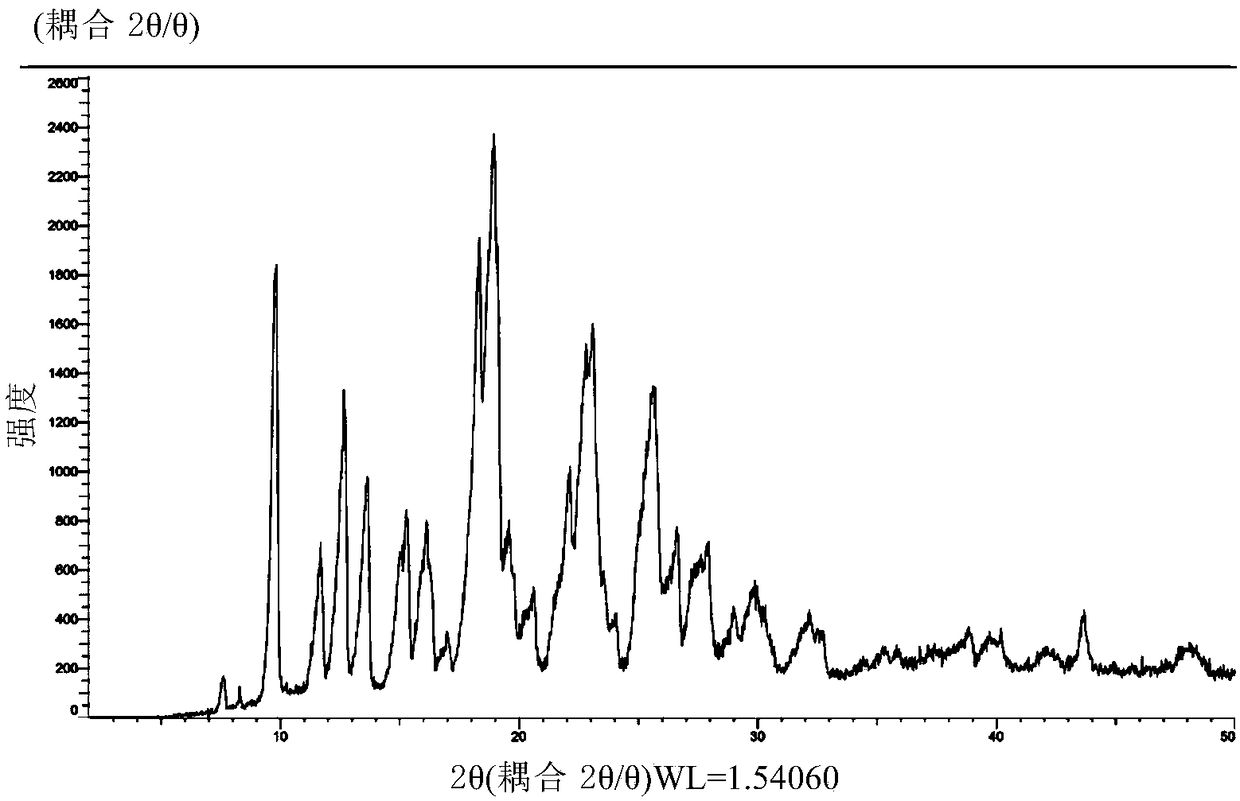
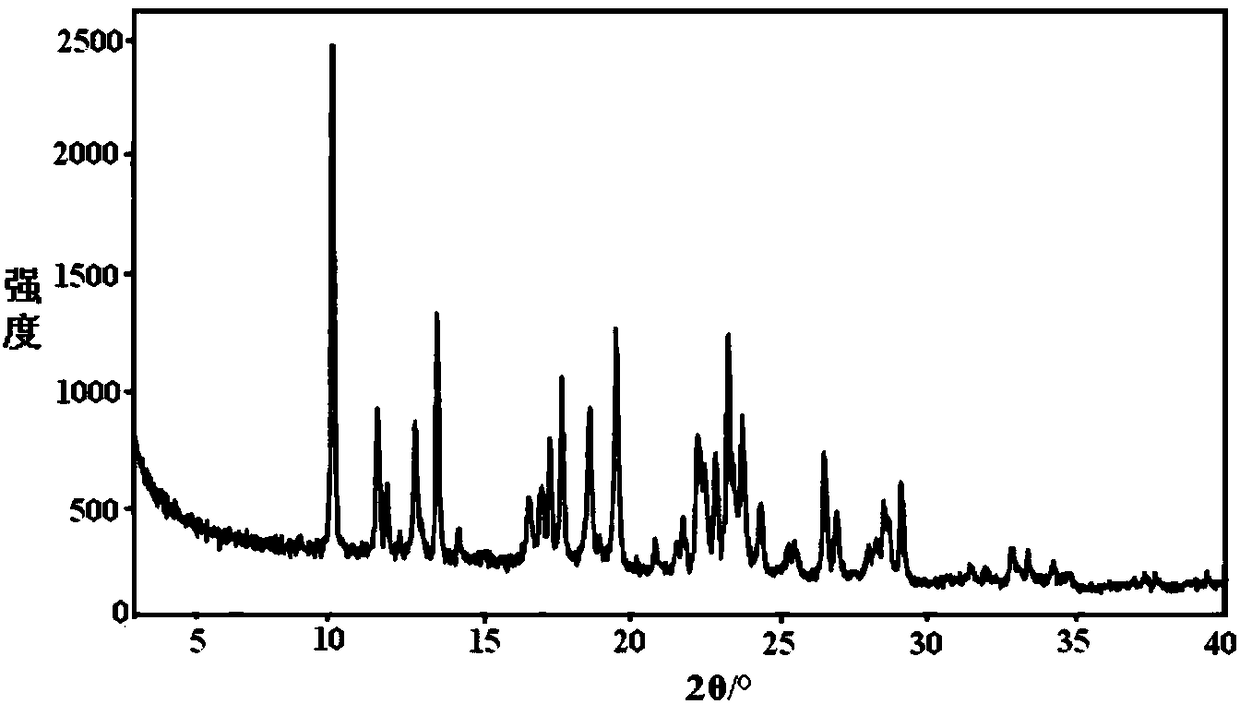
[0133] figure 1 is the XRD pattern of the obtained crystal, from figure 1 Visible: under powder X-ray diffraction, the crystal has 2θ at 9.8±0.2°, 11.7±0.2°, 12.7±0.2°, 15.3±0.2°, 18.3±0.2°, 19.0±0.2°, 23.1±0.2°, 25.6± There is a characteristic peak at 0.2°.
[0134] Further, under the powder X-ray diffraction, the crystal form C of idelalix of the present invention has 2θ of 9.8±0.2°,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com