Novel crystal form of pemetrexed disodium and preparation method thereof
A pemetrexed diacid and crystal form technology, applied in the field of medicinal chemistry, can solve the problems of unfavorable sample storage and transportation, excessive solvent residue in finished products, and low yield of crystal form A preparation, and achieve strong operability, containing Low water volume, easy to filter and dry effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1: Preparation of new crystal form X of pemetrexed diacid
Embodiment 11
[0050] At 80°C, 10g of pemetrexed diacid raw material (regardless of its form) was dissolved in a mixed solvent of 200mL DMF, 50mL ethanol and 30mL water to obtain a clear solution; the above solution was concentrated under reduced pressure at 60°C After at least a small amount of solid precipitation, stop the concentration and stirring for 1 hour; then slowly add 250 mL of water dropwise to the system at 50°C, then keep stirring for 1 hour; cool the system down to 5-10°C, keep stirring for 1 hour; filter (2 minutes for filtration) ) to collect the precipitated crystals, wash with water, and dry to obtain 8.2g crystals with a mass yield of 82%, an HPLC purity of 99.92%, and a moisture content of 7.2%.
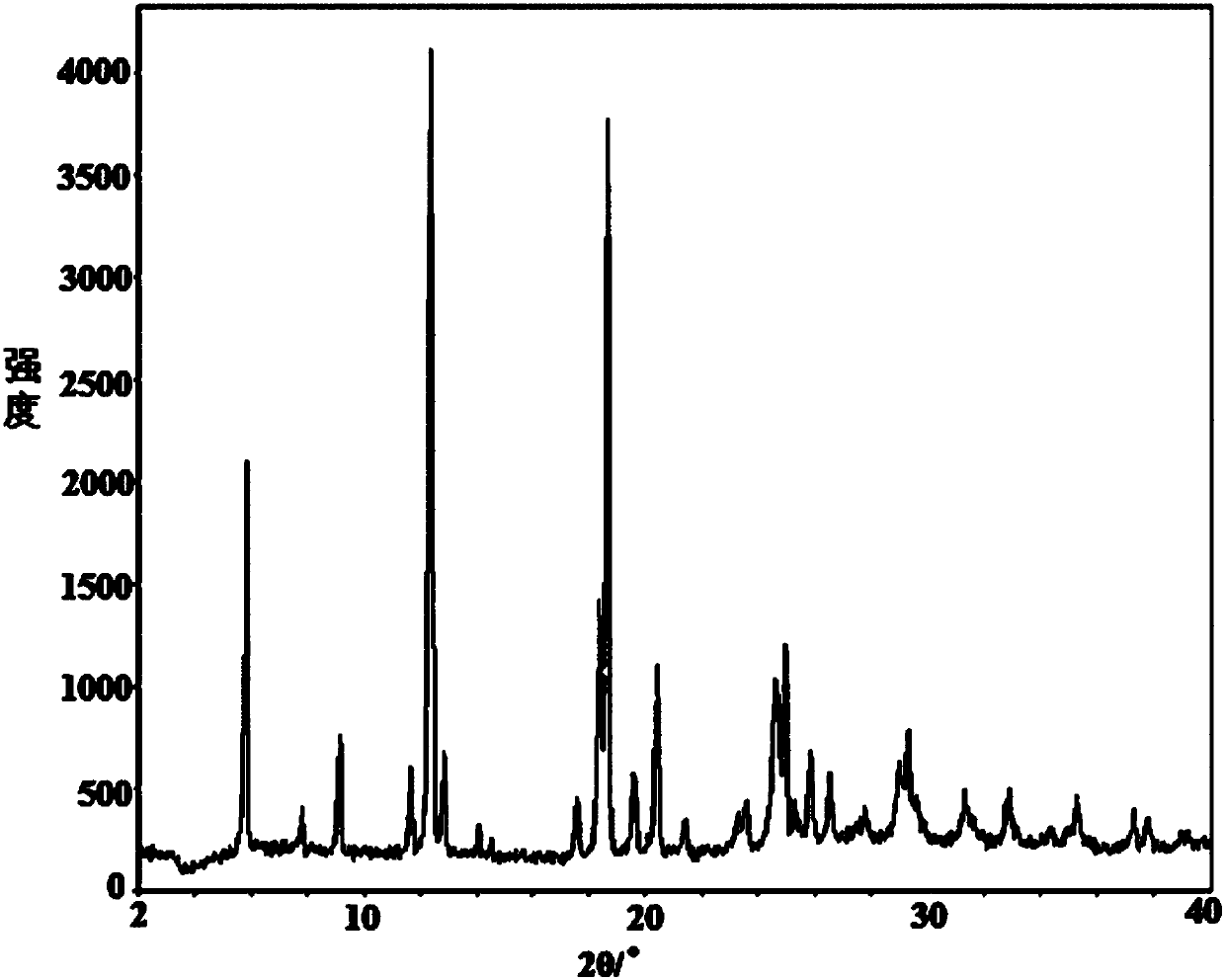
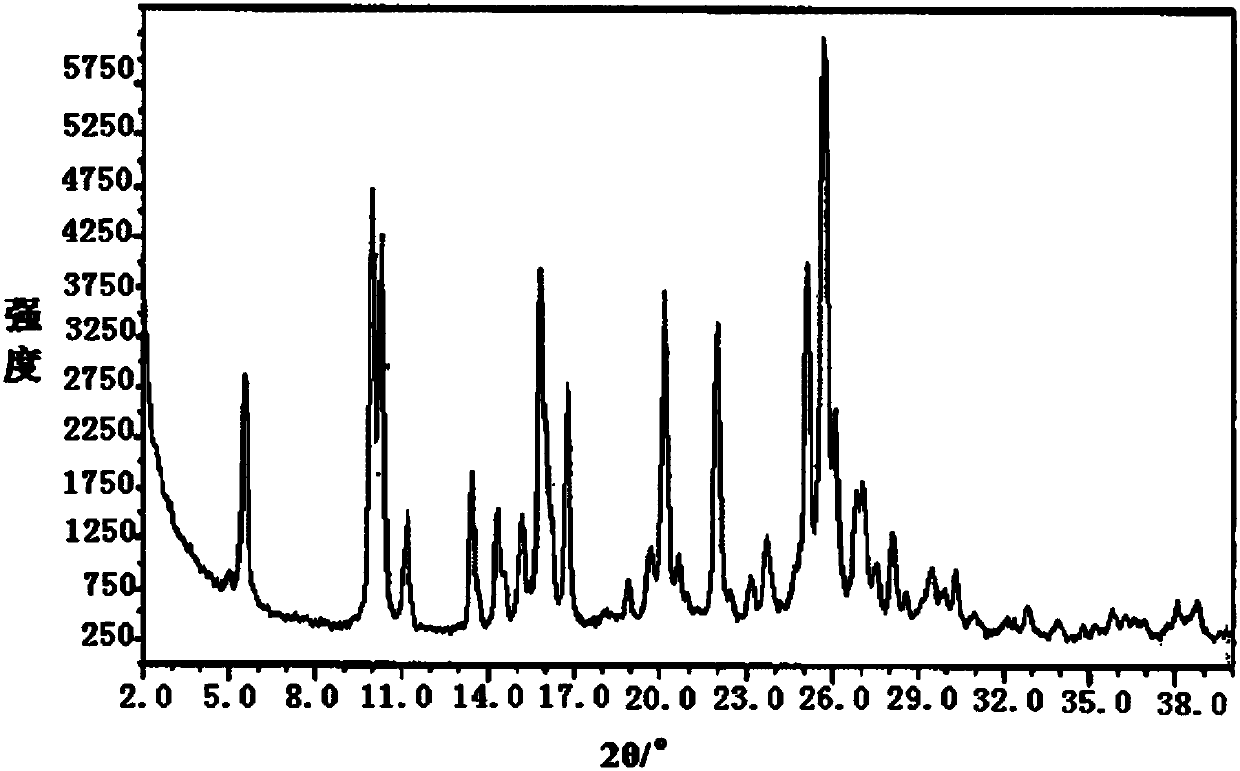
[0051] figure 1 is the XRD spectrum of the obtained crystal, by figure 1 It can be seen that under powder X-ray diffraction, the crystal has characteristic diffraction peaks at diffraction angles 2θ of 2θ of 7.82°, 9.20°, 12.84°, 18.70°, 19.56°, 24.60°, and 24.93°, and the tes...
Embodiment 12
[0058] At 60°C, 10g of pemetrexed diacid raw material (regardless of its form) was dissolved in a mixed solvent of 200mL DMF, 50mL methanol and 30mL water to obtain a clear solution; the above solution was concentrated under reduced pressure at 50°C After at least a small amount of solids were precipitated, stop the concentration and stirring for 1 hour; then slowly add 200 mL of water dropwise to the system at 50 ° C, and then keep stirring for 0.5 h; cool the system to 5-10 ° C, keep stirring for 1 h; filter (1.5 hours for filtration) Minutes) collected the precipitated crystals, washed with water, and dried to obtain 8.5 g of crystals with a mass yield of 85%, an HPLC purity of 99.93%, and a water content of 8.1%.
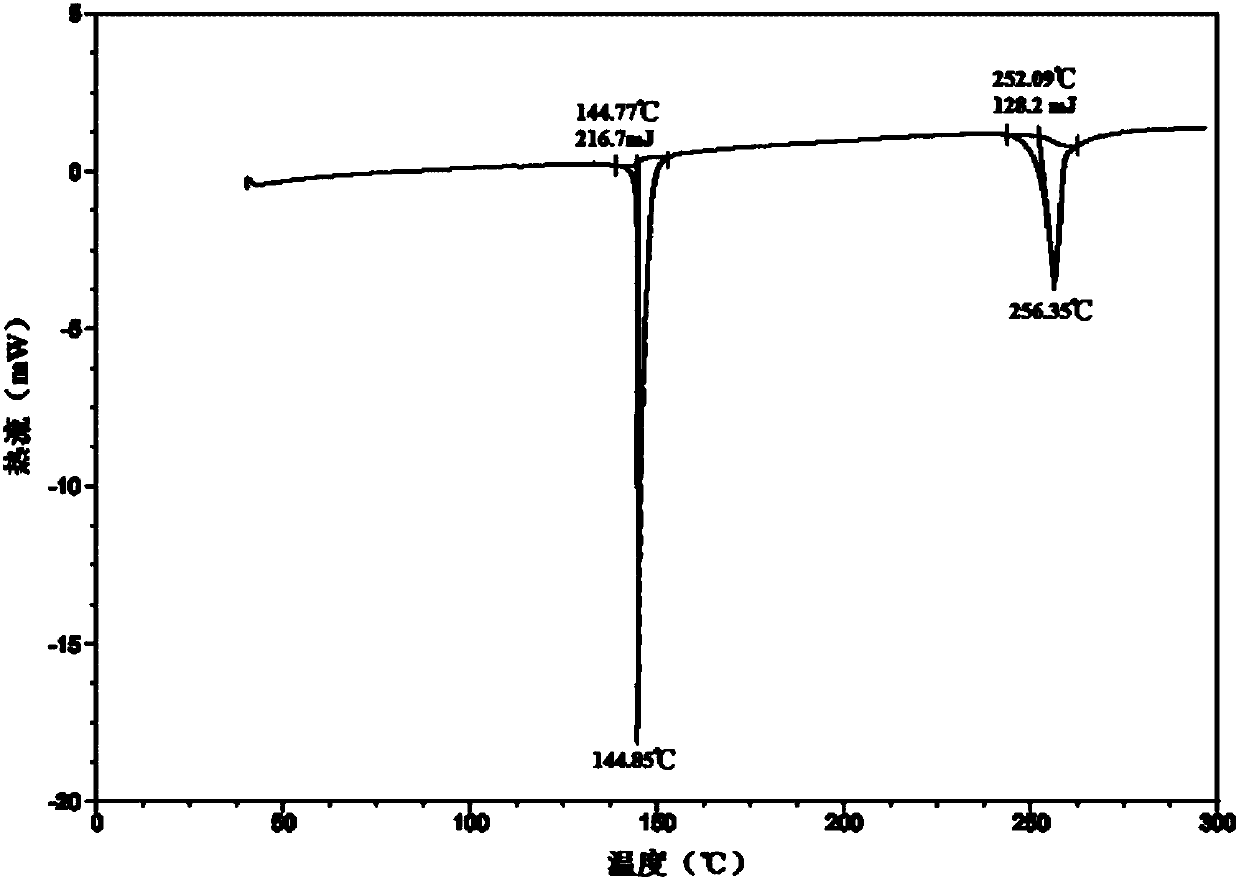
[0059] After determination and analysis, the obtained crystals have figure 1 Shown XRD spectrogram feature and figure 2 The characteristics of the DSC spectrum shown in this example indicate that the crystals obtained in this example are also the new crystal f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com