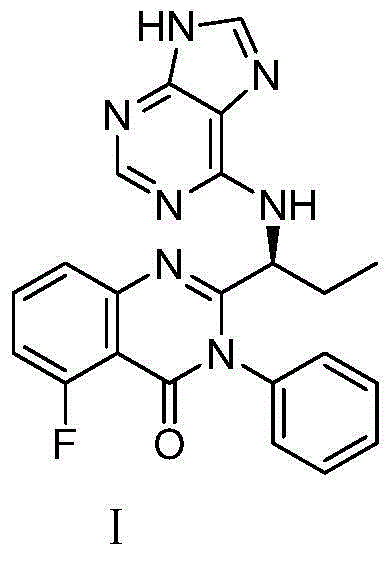
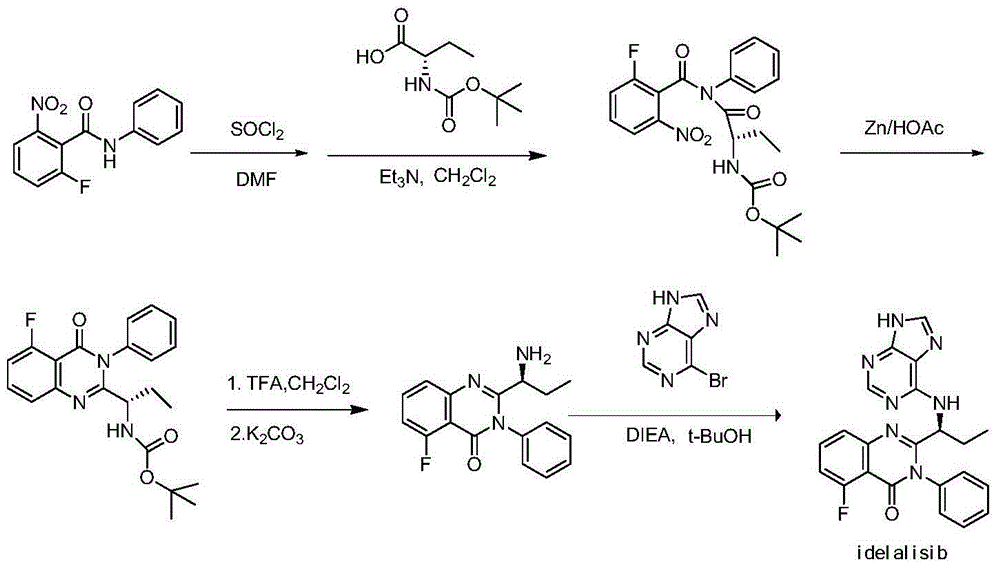
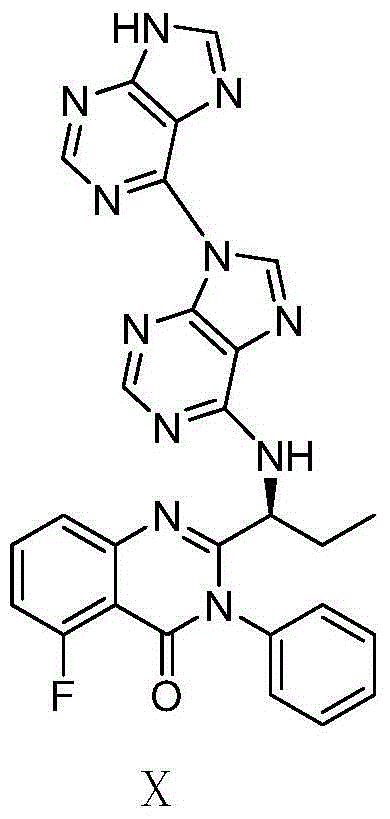
Preparation method of Idelalisib
A compound and reaction technology, applied in the field of preparation of Idelalisib, to achieve the effects of easy availability, mild reaction conditions and high production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Example 1 (S)-N-[2-[[3-fluoro-2-[(phenylamino)carbonyl]phenyl]amino]-1-ethyl-2-oxoethyl]-carbamic acid tertiary Preparation of butyl ester (compound of formula IV)
[0059] Dissolve N-BOC-L-2-aminobutyric acid (compound of formula III, 64g, 2eq) and N-methylmorpholine (35g, 2.2eq) in tetrahydrofuran (240mL), add isobutyl chloroformate dropwise at 0°C Ester (43g, 2.2eq), then dropwise into 2-amino-6-fluoro-N-phenylbenzamide (compound of formula II, 36g, 1eq) in tetrahydrofuran (300mL) solution, react at 60°C for 4 hours, and the reaction ends Then add saturated sodium bicarbonate solution, separate layers, take the lower aqueous solution and extract with ethyl acetate, wash the organic layer with saturated sodium chloride solution, concentrate to an oily substance, add n-hexane to make slurry and separate out 58g solid, yield: 90%, purity : 99% (area normalization method).
[0060] 1 HNMR (300MHz, CD 3 OD): δ H 8.08(1H, J=8.13),7.71(2H,J=7.98),7.48(1H,J=8.22),7.34(2...
Embodiment 2
[0063] Example 2 Preparation of (S)-2-(1-amino-propyl)-5-fluoro-3-phenyl-3H-quinazolin-4-one (compound of formula V)
[0064] Dissolve the compound of formula IV (58g, 1eq) and iodine (36g, 1eq) in dichloromethane (675mL), add HMDS (89.5mL, 3eq), heat and reflux for 36 hours, and add 10% thio Sodium sulfate solution (500 mL) neutralized excess iodine, the organic layer was washed with water and saturated sodium chloride solution, dried over anhydrous sodium sulfate, concentrated under reduced pressure to an oil, dissolved in ethyl acetate, and cooled to precipitate 28.7 g of solid. Yield: 70%, purity: 98.9% (area normalization method).
[0065] 1 HNMR (300MHz, CDCl 3 ):δ H 7.68(1H,m),7.52(4H,m),7.27(2H,m),7.09(1H,m),3.41(1H,dd,J=2.28,7.38),1.81(3H,m),1.50( 1H,m), 0.80 (3H,t,J=7.35).
[0066] 13 CNMR (75MHz, CDCl 3 ):δ C 161.4 (J C-F =264.8), 161.2, 149.5, 136.2, 134.7 (J C-F =10.5), 129.9, 129.7, 129.4, 129.0, 128.3, 123.1 (J C-F =4.5),113.2(J C-F =21.0), 54.3, 30....
Embodiment 3
[0068] Example 3 Preparation of 6-chloro-9-(tetrahydro-2H-pyran-2-yl)-9H-purine (compound of formula VIII)
[0069] Dissolve 6-chloropurine (19.9g, 1eq) in dichloromethane (199mL), add dropwise 2-hydropyran (16.3g, 1.5eq), react at 30°C for 2 hours, add the reaction solution into water, and separate layers , the organic layer was washed with saturated sodium chloride solution, dried over anhydrous sodium sulfate, and concentrated to dryness under reduced pressure to obtain 27.7 g of solid, which was scraped off for later use. Yield: 90%, purity: 96% (area normalization method).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com