Crystal form, pharmaceutical composition, preparation method and use of Idelalis
A technology of composition and crystal form, applied in the direction of drug combination, organic chemical method, active ingredient of heterocyclic compound, etc., can solve the problems that the preparation method needs to be continuously improved
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0117] Example 1: Preparation of Idelaris Form R
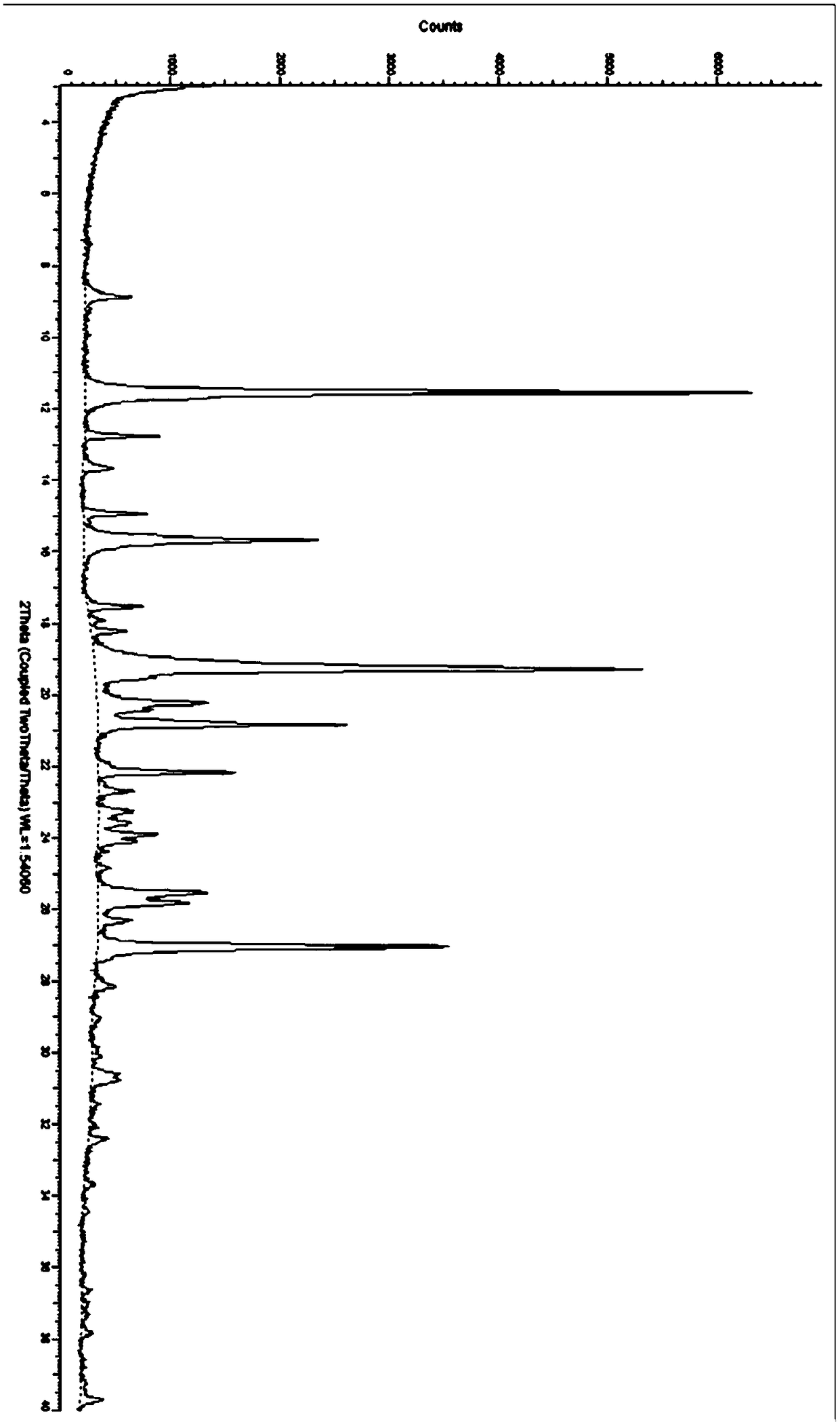
[0118] In a 3L glass bottle, add 200 g of idelaris as an amorphous solid, add 200 ml of dichloromethane, and 50 ml of absolute ethanol, stir mechanically, and heat to reflux at 40°C. After starting to reflux, 1.6 L of acetonitrile was added dropwise, while continuing to raise the temperature to 55°C. The dropwise addition was completed in 5 minutes, and stirring was continued for 30 minutes. Rapid cooling down to 5°C. A large amount of product was precipitated, and the stirring was continued for 1 hour, and the product was obtained by filtration, and the solid was dried in a blast drying oven at 55° C. for 8 hours to obtain 190 g of Idelalis crystal form R, and its X-ray powder diffraction pattern was as follows: figure 1 shown.
Embodiment 2
[0119] Example 2: Preparation of Idelaris Form R
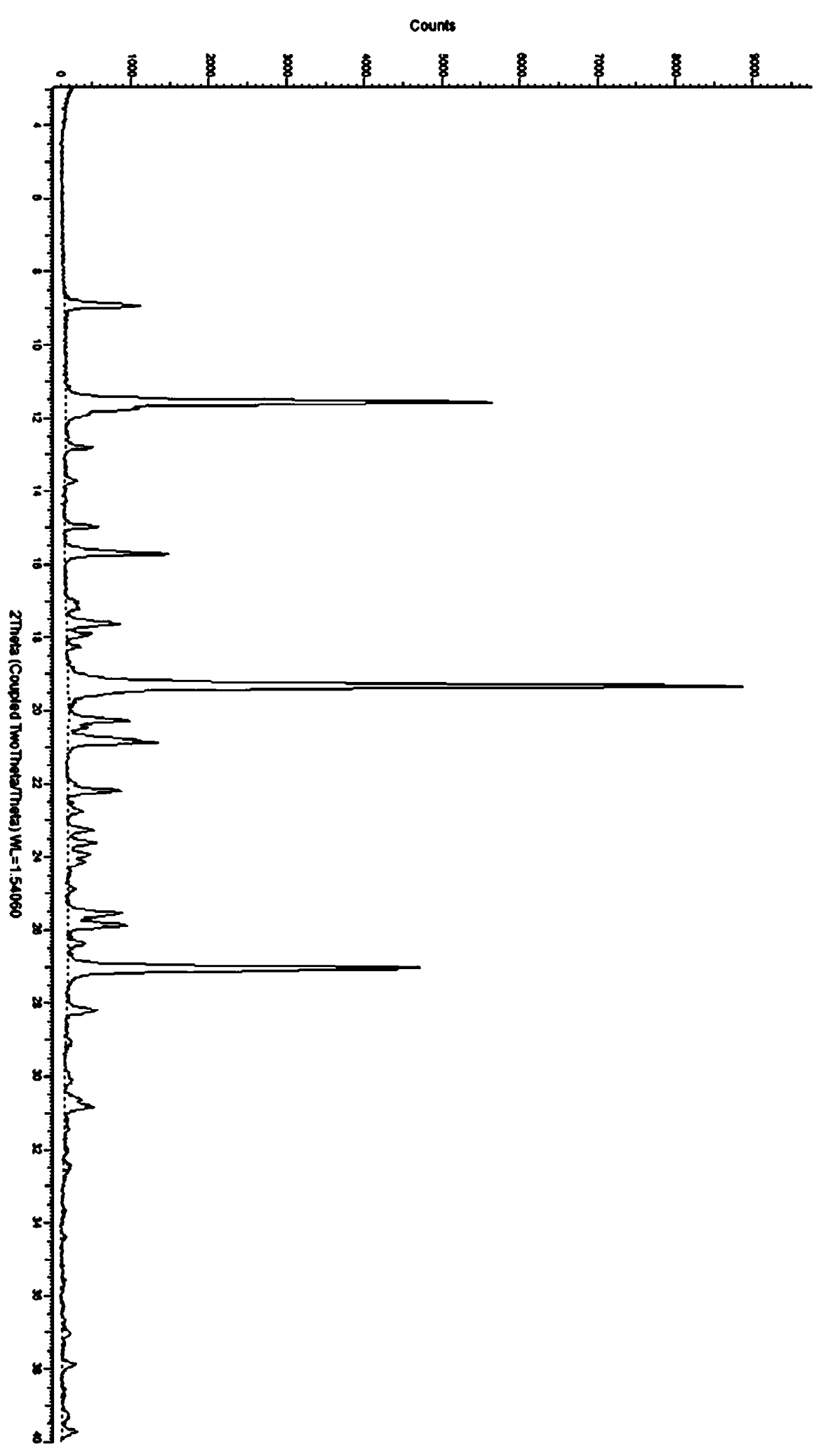
[0120] In a 3L glass bottle, add 200g of idelaris amorphous solid, add 200ml of dichloromethane, 50ml of absolute ethanol, stir magnetically, and heat to 40°C. 2 L of acetonitrile was added dropwise while continuing to raise the temperature to 55°C. The dropwise addition was completed in 5 minutes, and stirring was continued for 30 minutes. Rapid cooling down to 0°C. A large amount of product was precipitated, and the stirring was continued for 1 hour, and the product was obtained by filtration, and the solid was dried in a blast oven at 55° C. for 8 hours to obtain 183 g of idelaris crystal form R, whose X-ray powder diffraction pattern was as follows: figure 2 shown.
Embodiment 3
[0121] Example 3: Preparation of Idelaris Form R
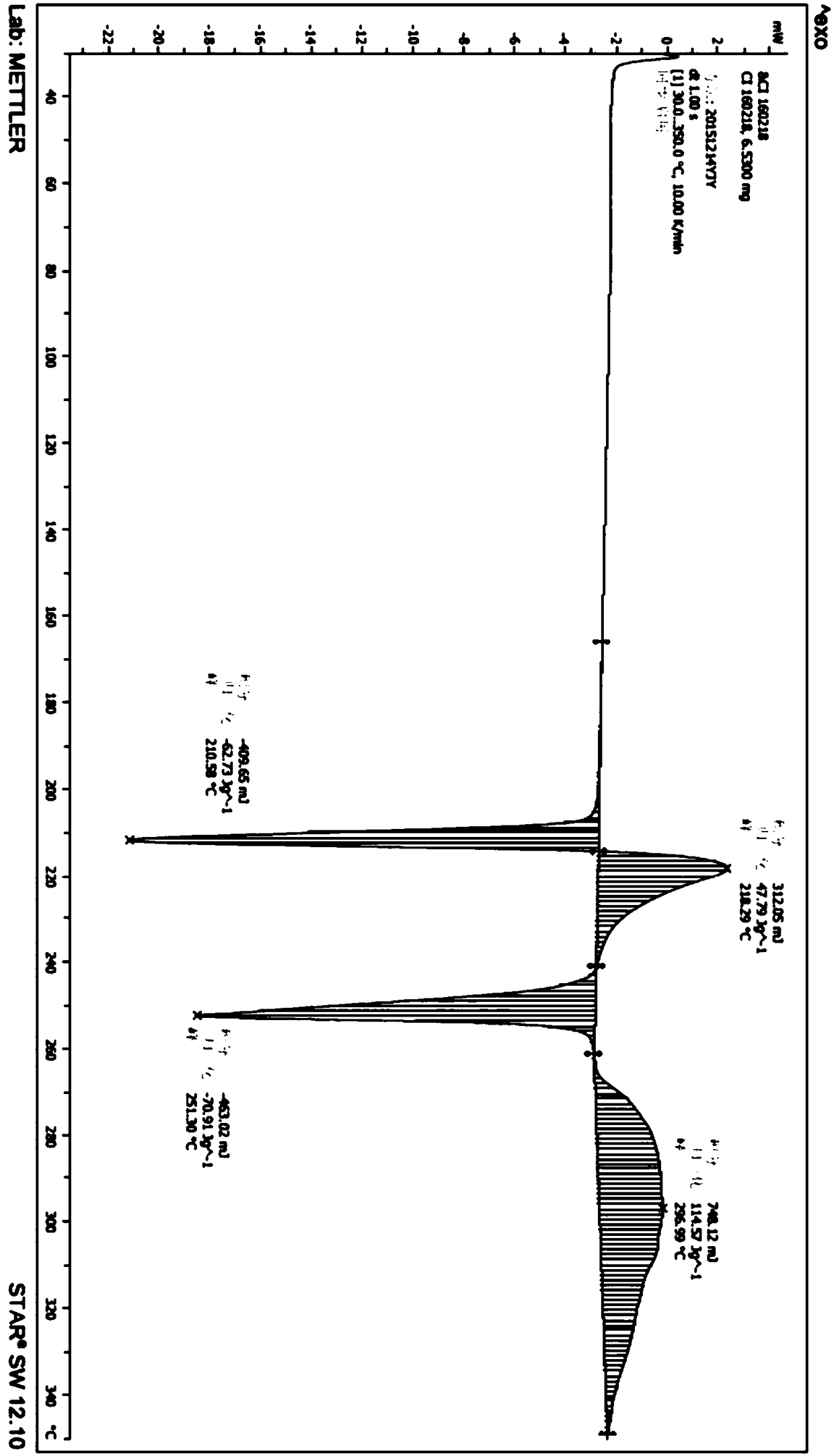
[0122] In a 5L glass bottle, add 500g of idelaris amorphous solid, add 500ml of dichloromethane, 75ml of absolute ethanol, stir mechanically, cool to 0°C, add dropwise 4L of acetonitrile, dropwise add in 10 minutes, continue to Stir at 0°C for 30 minutes. The product was obtained by filtration, and the solid was baked in a blast drying oven at 55°C for 8 hours to obtain 462g of Idelaris crystal form R, and its X-ray powder diffraction pattern was as follows: Figure 9 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com