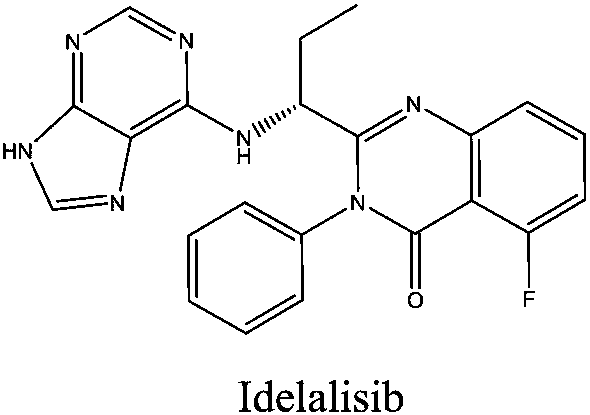
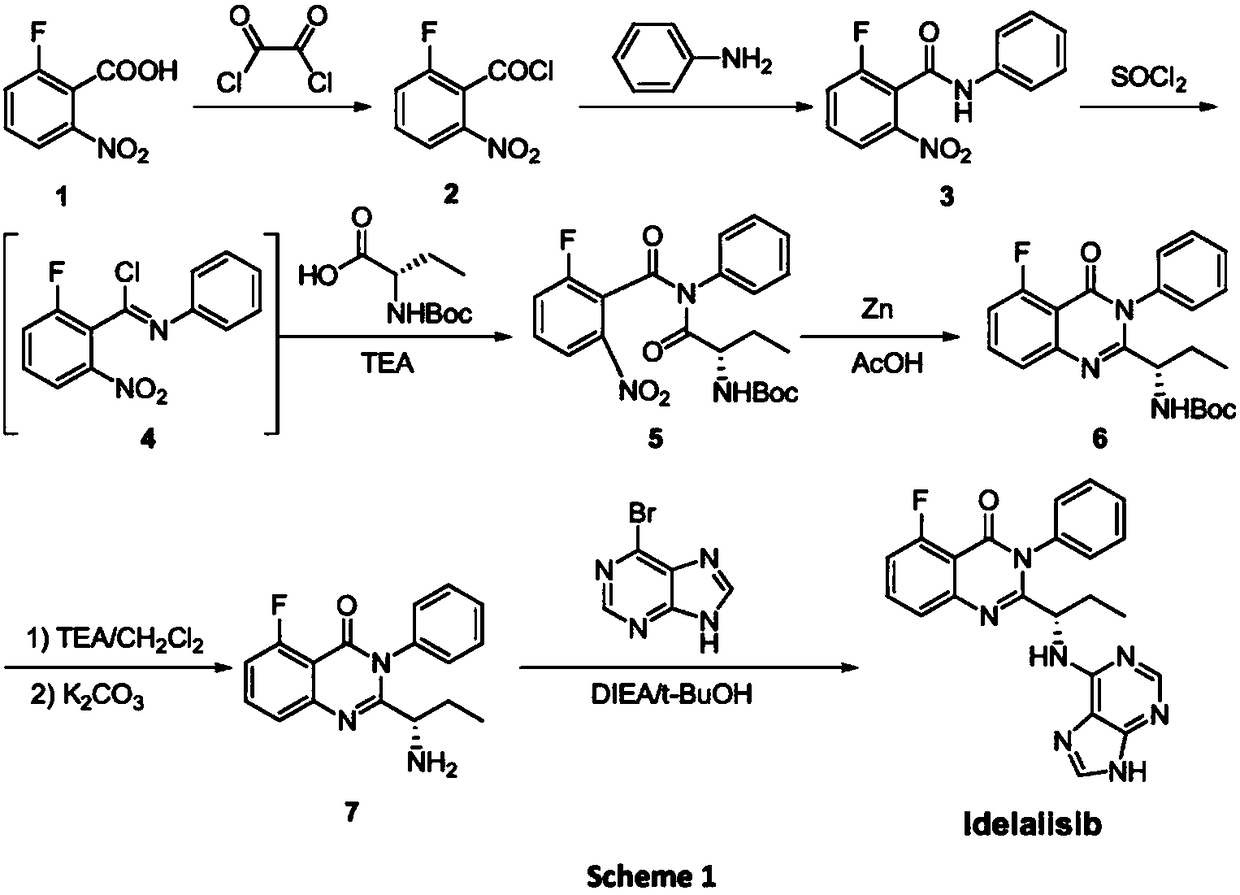
Novel idelalisib preparation method
A synthesis method and Lewis acid technology, which are applied in the fields of organic chemistry, drug combination, antitumor drugs, etc., can solve the problems of intractable zinc acetate, difficult product extraction, and difficult purification, saving time and labor costs, and avoiding synthesis conditions. Harsh, easy post-processing effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
[0064] Implementation Case 1. Intermediate C 1 Synthesis
[0065]
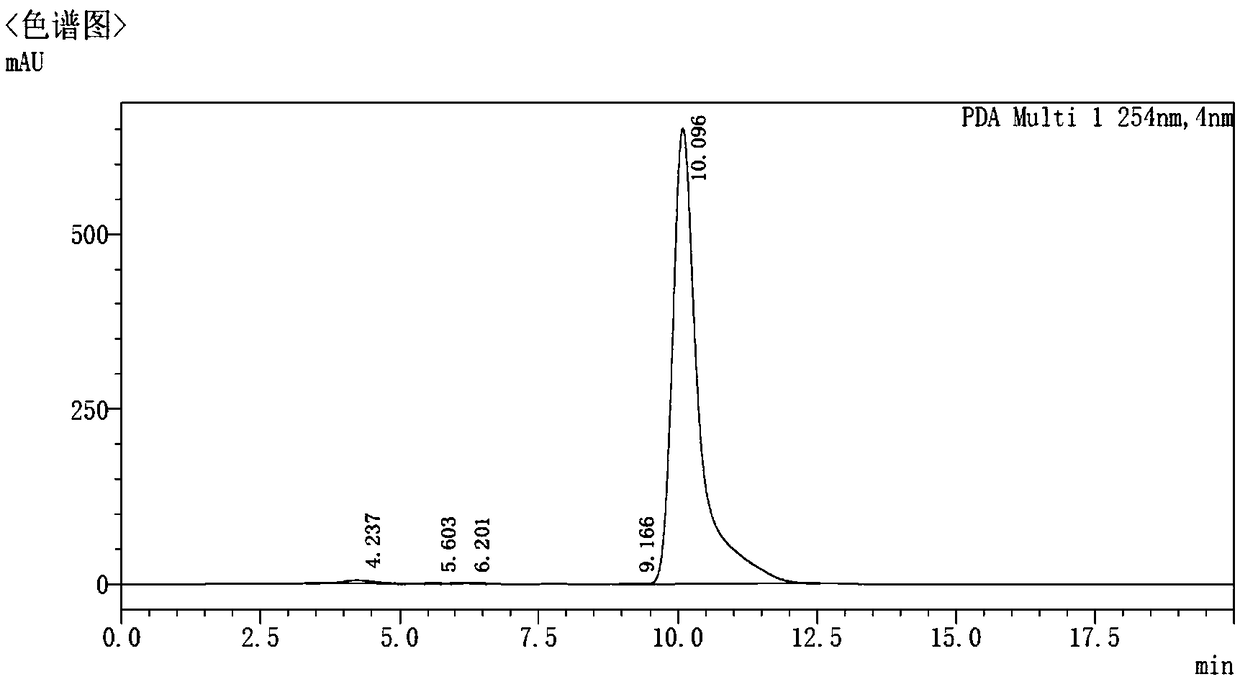
[0066] L-2-aminobutyric acid ethyl ester (A 1 , 3.3g, 25mmol), Compound B 1 (5.16g, 20.3mmol) and diisopropylethylamine (5.6mL, 33.7mmol) were dissolved in tert-butanol / ethanol (20mL / 10mL), N 2 Protection, reflux reaction at 90°C for 30h, HPLC monitoring reaction to B 1 disappear. The reaction liquid was cooled to ambient temperature, the solvent was distilled off under reduced pressure, washed with water, filtered, and the filter cake was dried and recrystallized from acetone to obtain 5.6 g of solids with a yield of 79.1% (HPLC: 95.2%).
Embodiment example 2
[0067] Example 2. Intermediate C 1 Synthesis
[0068] L-2-aminobutyric acid ethyl ester (A 1 , 6.6g, 50mmol), Compound B 1 (10.32g, 40.6mmol) and triethylamine (6.81g, 67.4mmol) were dissolved in isopropanol (40mL), N 2 Protection, reflux reaction at 90°C for 30h, HPLC monitoring reaction to B 1 disappear. The reaction solution was cooled to ambient temperature, the solvent was distilled off under reduced pressure, washed with water, filtered, and the filter cake was dried and recrystallized from acetone to obtain 10.21 g of solids with a yield of 72.1% (HPLC: 95.8%).
Embodiment example 3
[0069] Implementation Case 3. Intermediate C 2 Synthesis
[0070]
[0071] Methyl L-2-aminobutyrate (A 2 , 5.85g, 50mmol), compound B 1 (10.32g, 40.6mmol) and triethylamine (6.81g, 67.4mmol) were dissolved in tert-butanol (40mL), N 2 Protection, reflux reaction at 90°C for 30h, HPLC monitoring reaction to B 1 disappear. The reaction liquid was cooled to ambient temperature, the solvent was distilled off under reduced pressure, washed with water, filtered, and the filter cake was dried and recrystallized from acetone to obtain 11.23 g of solids with a yield of 82.5% (HPLC: 97.2%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com