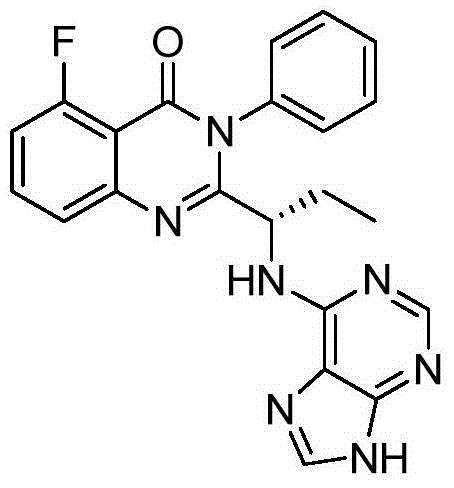
Preparation method of (S)-2-(1-amino-propyl)-5-fluoro-3-phenyl-3H-quinazoline-4-one
A quinazoline, phenyl technology, applied in the field of preparation of -2--5-fluoro-3-phenyl-3H-quinazolin-4-one, can solve the problem of low yield, low overall yield, Purity is not good and other problems, to achieve the effect of mild reaction conditions, high yield and stable reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-2
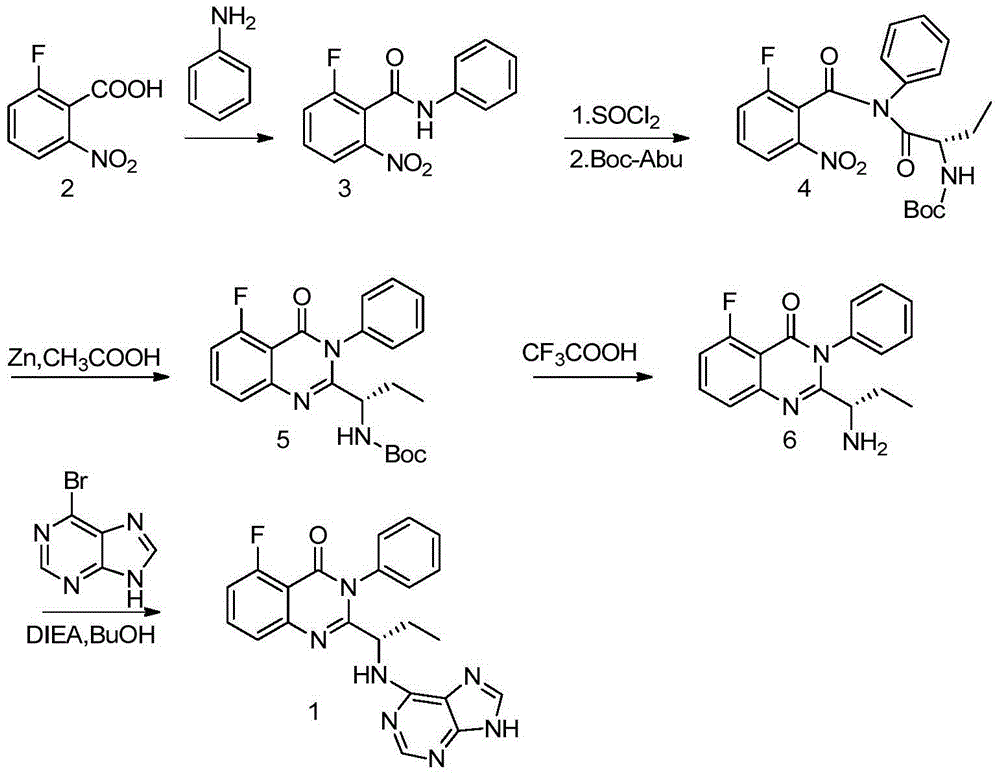
[0023] Example 1-2: Preparation of 2-fluoro-6-nitro-N-phenyl-benzamide formula (II)
[0024] 2-Fluoro-6-nitrobenzoic acid (18.5g), DMF (1ml) and dichloromethane (100ml) were mixed and stirred, and a dichloromethane solution (60ml) containing oxalyl chloride (19g) was added dropwise, and the reaction was stirred at room temperature After 2 hours, concentrate to give an orange solid syrup. The above slurry was dissolved in anhydrous dioxane (16ml), and slowly added dropwise to aniline (9ml) and sodium bicarbonate (16.8g) in dioxane (40) and water (40) at 6°C. After the addition, stir at room temperature for half an hour, add water (250ml), a solid is produced, collect the solid by filtration, wash with water, and obtain the title compound 2-fluoro-6-nitro-N-phenyl-benzene Formamide 25.6g, yield 98.4%. 1 H NMR (400MHz, CDCl 3 )δ8.01(d,J=8.2Hz,1H),7.70-7.60(m,,4H),7.41(t,J=7.8Hz,2H),7.23(t,J=7.4Hz,1H).ESI -MS(m / z):261[M+H] +
[0025] 2-Fluoro-6-nitrobenzoic acid (18.5g), DMF...
Embodiment 3-4
[0026] Example 3-4: Preparation of 2-amino-6-fluoro-N-phenyl-benzamide formula (Ⅲ)
[0027] A mixture of 2-amino-6-fluoro-N-phenyl-benzamide (13g), Pd / C (1g) and ethyl acetate (100ml) was hydrogenated at 50°C for four hours, filtered and concentrated to dryness to obtain the title Compound 2-amino-6-fluoro-N-phenyl-benzamide 11.1 g, yield 96.5%. 1 H NMR (400MHz, CDCl 3 )δ8.33(d,J=15.5Hz,1H),7.61(d,J=7.6Hz,2H),7.43–7.35(m,2H),7.21–7.12(m,2H),6.51(d,J =8.3Hz,1H),6.43(ddd,J=13.0,8.1,1.0Hz,1H),5.97(s,2H).ESI-MS(m / z):231[M+H] +
[0028] 2-Amino-6-fluoro-N-phenyl-benzamide (13g), Pd / C (0.8g) and ethyl acetate (100ml) were mixed and hydrogenated at 50°C for six hours, filtered and concentrated to dryness to obtain 10.5 g of the title compound 2-amino-6-fluoro-N-phenyl-benzamide, yield 91.3%.
Embodiment 5-6
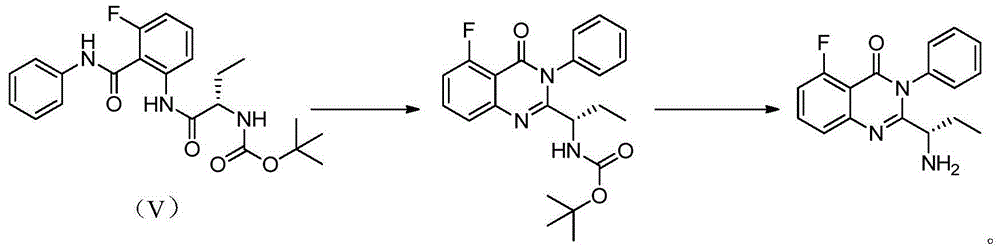
[0029] Example 5-6: Preparation of (S)-2-{[3-fluoro-2-[(phenylamino)carbonyl]phenyl]amino-1-ethyl}-carbamate isobutyl ester formula (V)
[0030] Dissolve BOC-L-2-aminobutyric acid (20.3g) and N-methylmorpholine (11.2g) in anhydrous THF (120ml), and add isobutyl chloroformate (13.7g ) solution in anhydrous THF (40ml), dropwise, stirred at -15°C for 1 hour, then added dropwise an anhydrous solution containing 2-amino-6-fluoro-N-phenyl-benzamide (11.5g) THF (40ml) solution, dropwise, react at this temperature for half an hour, slowly rise to room temperature, react for 1 hour, filter out the solid, the filtrate is heated to reflux for 3.5 hours, concentrate the reaction solution, add ethyl acetate (150ml) and Water (200ml), separated, the aqueous layer was extracted with ethyl acetate (100ml×2), the organic layers were combined, washed with water, washed with saturated brine, dried, filtered, and concentrated to obtain 19.2g of crude product, which was recrystallized with isoprop...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com