Preparation method of Idelalisib intermediate
A technology for idelaris and intermediates, which is applied in the field of preparation of idelaris intermediates, can solve the problems of low yield, cumbersome preparation methods, and low optical purity, and achieve high yield and simple post-processing Ease of application and high optical purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
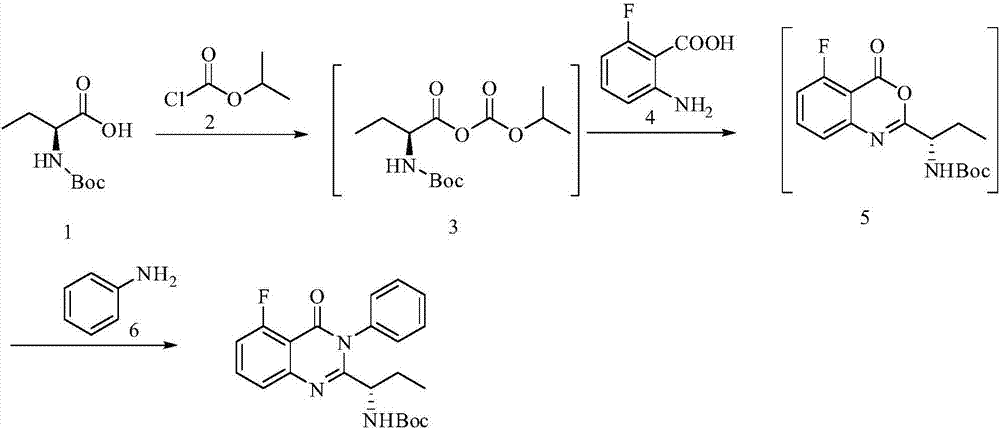
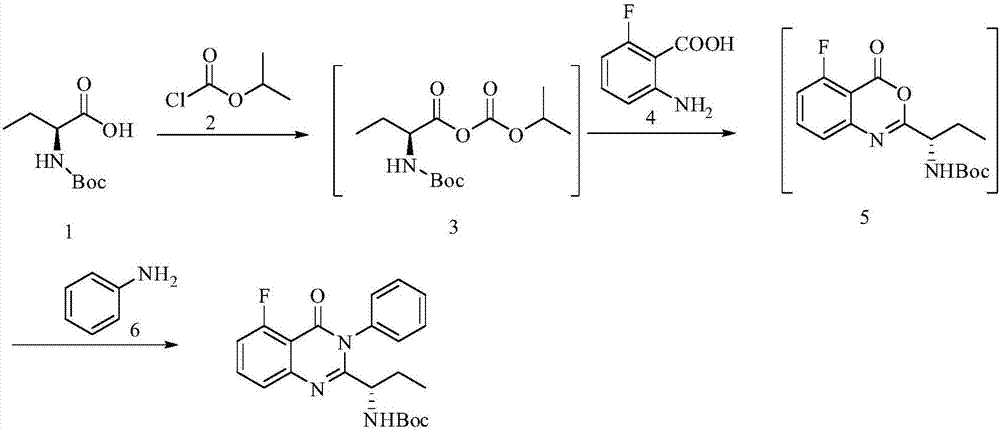
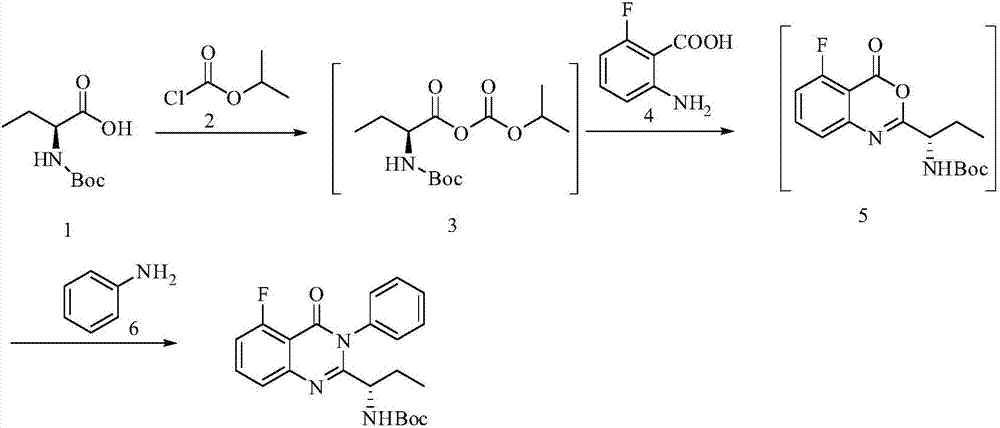
[0029] Example 1 (S)-(1-(5-fluoro-4-carbonyl-3-phenyl-3,4-dihydroquinazolin-2-yl)propyl)carbamic acid tertiary Preparation of butyl ester
[0030]
[0031] Add N,N-dimethylformamide (720ml) into the reaction flask, add N-Boc-L-2-aminobutyric acid (A, 203g, 1mol), stir and cool to 0-10°C; at this temperature, Add N-methylmorphine (151g, 1.5mol) dropwise; then add isopropyl chloroformate (122.5g, 1mol) dropwise, stir for 1h after the addition is complete; finally add 2-amino-6-fluorobenzoic acid dropwise (155g, 1mol) of N,N-dimethylformamide (120ml) mixed solution, stirred for 5h, added aniline (139.5g, 1.5mol), heated to 50°C, reacted for 14h, after the reaction was completed, added water (5000ml ), the crude product was precipitated and recrystallized with isopropanol to obtain off-white solid (S)-(1-(5-fluoro-4-carbonyl-3-phenyl-3,4-dihydroquinazolin-2-yl ) Propyl) tert-butyl carbamate 365.2 g (92%), EE value: 99.7%.
[0032] 1H NMR (400MHz, 25℃, DMSO-d6): 7.83(td, J=...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com