O-fluorine o-imidogen benzoic acid intermediate compound as well as preparation method and application thereof
A technology of o-iminobenzoic acid and compounds, which is applied in the field of synthesis of pharmaceutical intermediates, can solve the problems of high ee% value and high purity of intermediates, increase the difficulty of product separation, increase the complexity of reactions, etc., and achieve easy formation of final product, avoid reaction by-products, high reactivity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] In this example, the corresponding chirality is the compound of formula I of S (S)-2-((2-((tert-butoxycarbonyl)amino)-1-phenoxybutenyl)amino)-6-fluoro The concrete preparation of benzoic acid is as follows:
[0050]
[0051] Dissolve 10g (0.05mol) N-Boc-L-2-aminobutyric acid in an appropriate amount of 100mL toluene solvent, add 4g pyridine and 17g P(OPh) 3 Stir and mix evenly, continue to stir to activate the carboxylic acid completely, then add 8.5g of 2-amino-6-fluorobenzoic acid, heat up to 55°C to conduct the condensation reaction for 1.5 hours, and follow up to confirm that the reaction is complete; after the reaction, Add 1M hydrochloric acid to acidify the reaction solution, let it stand, separate layers, collect the organic layer, concentrate and remove the solvent to obtain 15.2 g of oil, which is (S)-2-((2-((tert-butoxycarbonyl)amino) -1-phenoxybutenyl)amino)-6-fluorobenzoic acid, the oil can be further purified by flash column separation to obtain pure p...
Embodiment 2
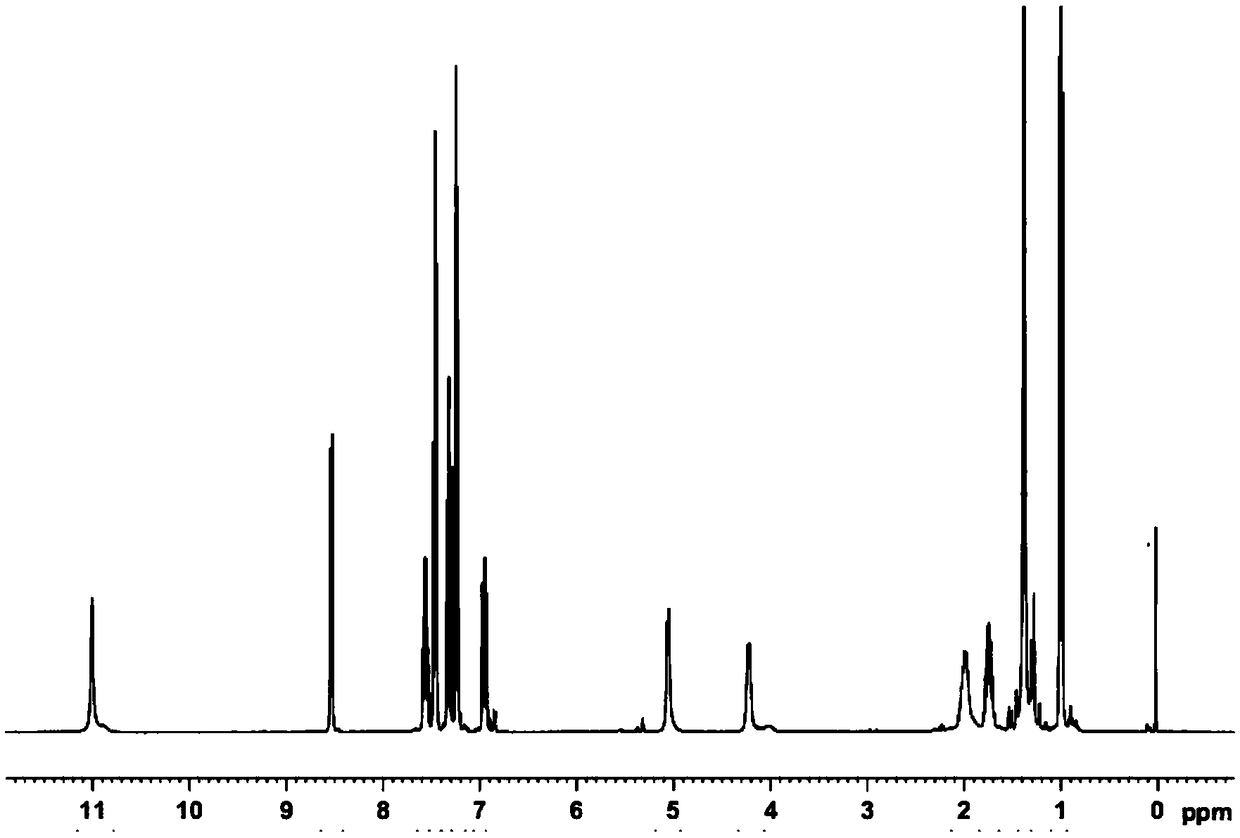
[0053] Dissolve 10g (0.05mol) N-Boc-L-2-aminobutyric acid in an appropriate amount of 150mL toluene solvent, add 6g pyridine and 16g P(OPh) 3 Stir and mix evenly, continue to stir to fully activate the carboxylic acid, then add 9.0 g of 2-amino-6-fluorobenzoic acid, raise the temperature to 60°C, and control the temperature at 60°C-65°C to carry out the condensation reaction for 1.0 hour, Follow up to confirm that the reaction is complete; after the reaction, add 1M hydrochloric acid to acidify the reaction solution, leave it to stand, separate layers, collect the organic layer and concentrate to remove the solvent to obtain an oil, which is (S)-2-((2-(( tert-butoxycarbonyl)amino)-1-phenoxybutenyl)amino)-6-fluorobenzoic acid, and the oil can be separated and purified by flash column to obtain pure product. The obtained (S)-2-((2-((tert-butoxycarbonyl)amino)-1-phenoxybutenyl)amino)-6-fluorobenzoic acid is carried out to corresponding structural analysis, the results show Its 1...
Embodiment 3
[0055] Dissolve 10g (0.05mol) N-Boc-L-2-aminobutyric acid in an appropriate amount of 150mL toluene solvent, add 7g pyridine and 18g P(OPh) 3 Stir and mix evenly, continue to stir to fully activate the carboxylic acid, then add 8.5 g of 2-amino-6-fluorobenzoic acid, raise the temperature to 75°C, and control the temperature at 75°C-80°C to carry out the condensation reaction for 1.0 hour, Follow up to confirm that the reaction is complete; after the reaction, add 1M hydrochloric acid to acidify the reaction solution, leave it to stand, separate layers, collect the organic layer and concentrate to remove the solvent to obtain an oil, which is (S)-2-((2-(( tert-butoxycarbonyl)amino)-1-phenoxybutenyl)amino)-6-fluorobenzoic acid, and the oil can be separated and purified by flash column to obtain pure product. The obtained (S)-2-((2-((tert-butoxycarbonyl)amino)-1-phenoxybutenyl)amino)-6-fluorobenzoic acid was subjected to structural analysis, and its 1H-NMR , 31 The P-NMR spectr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com