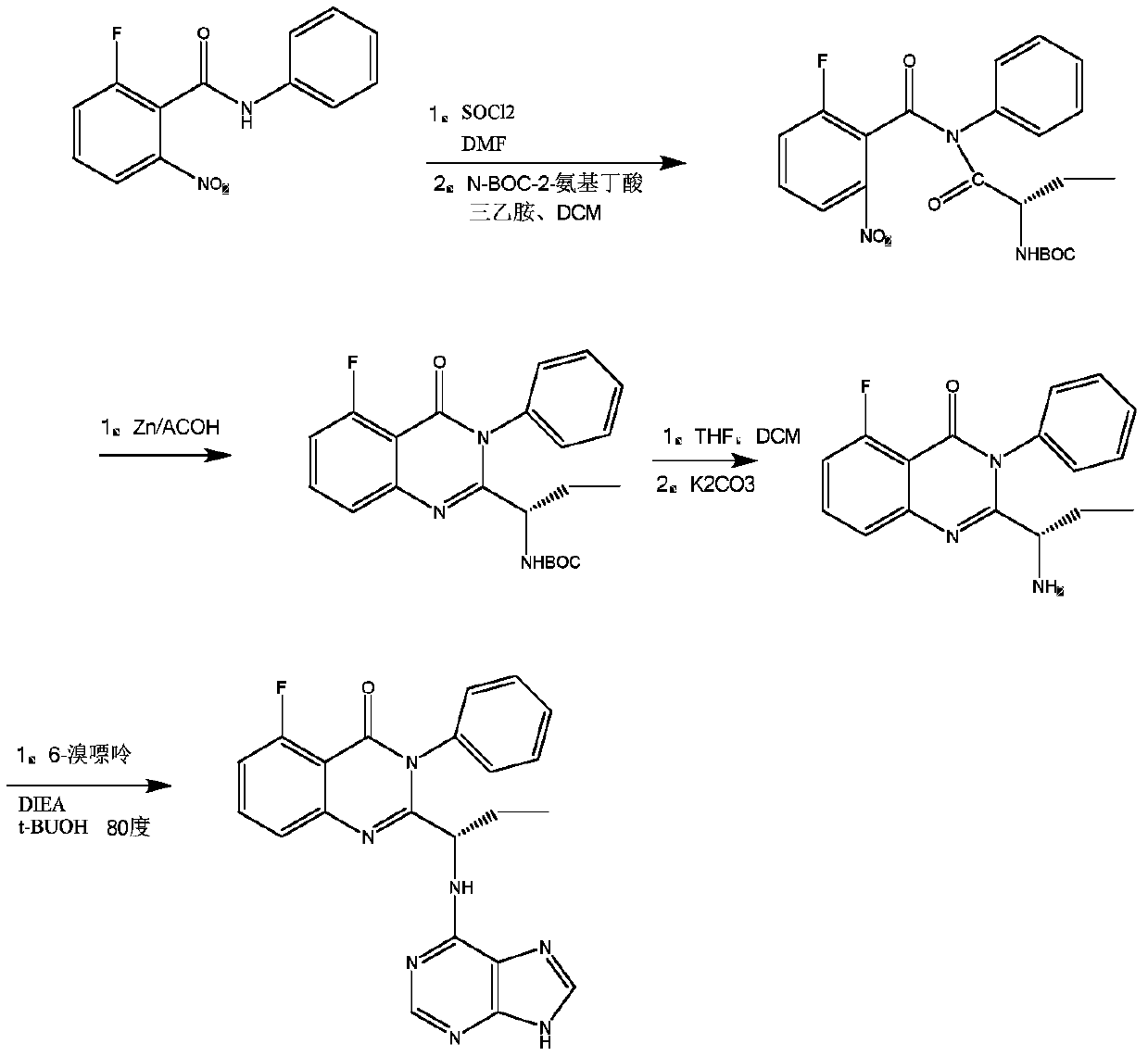
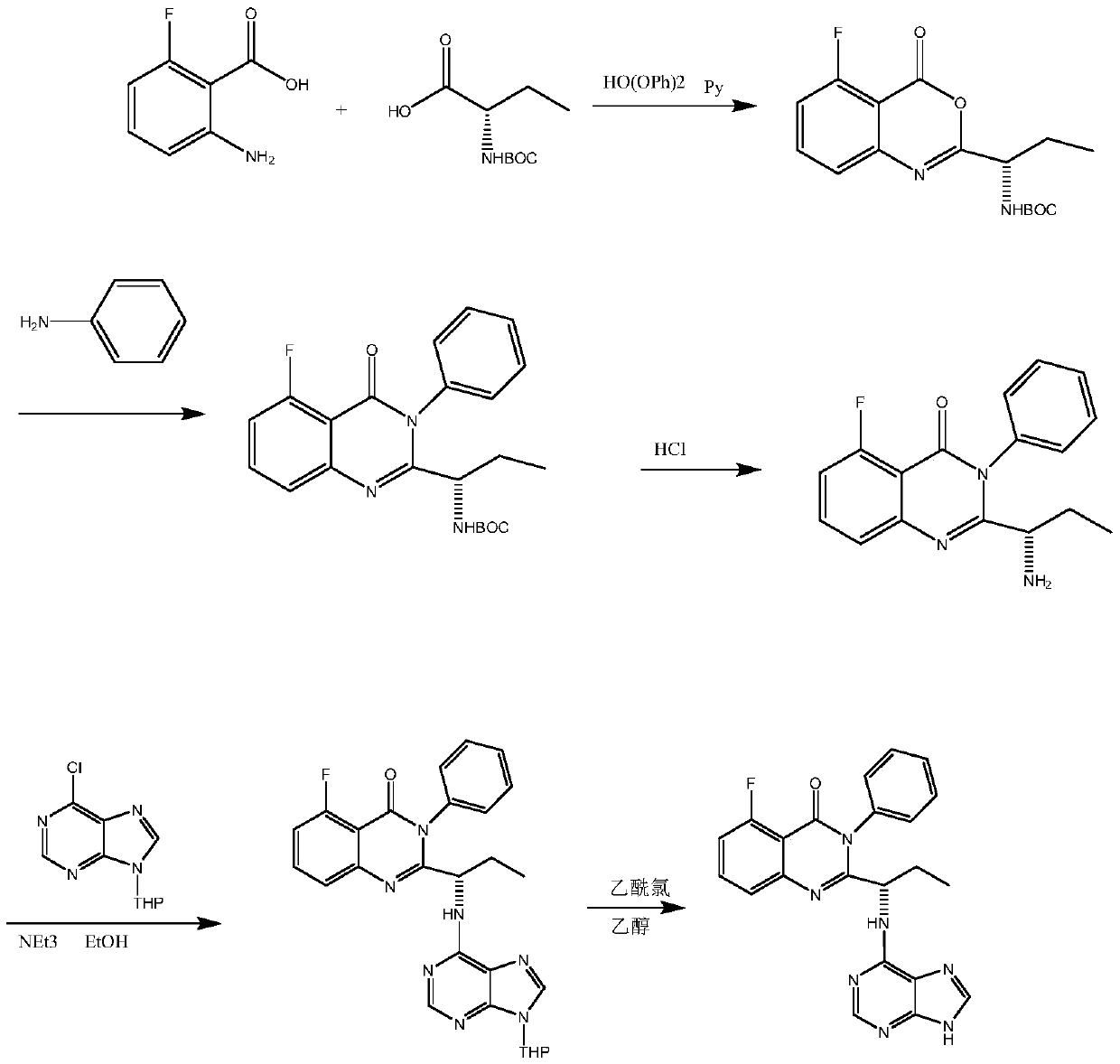
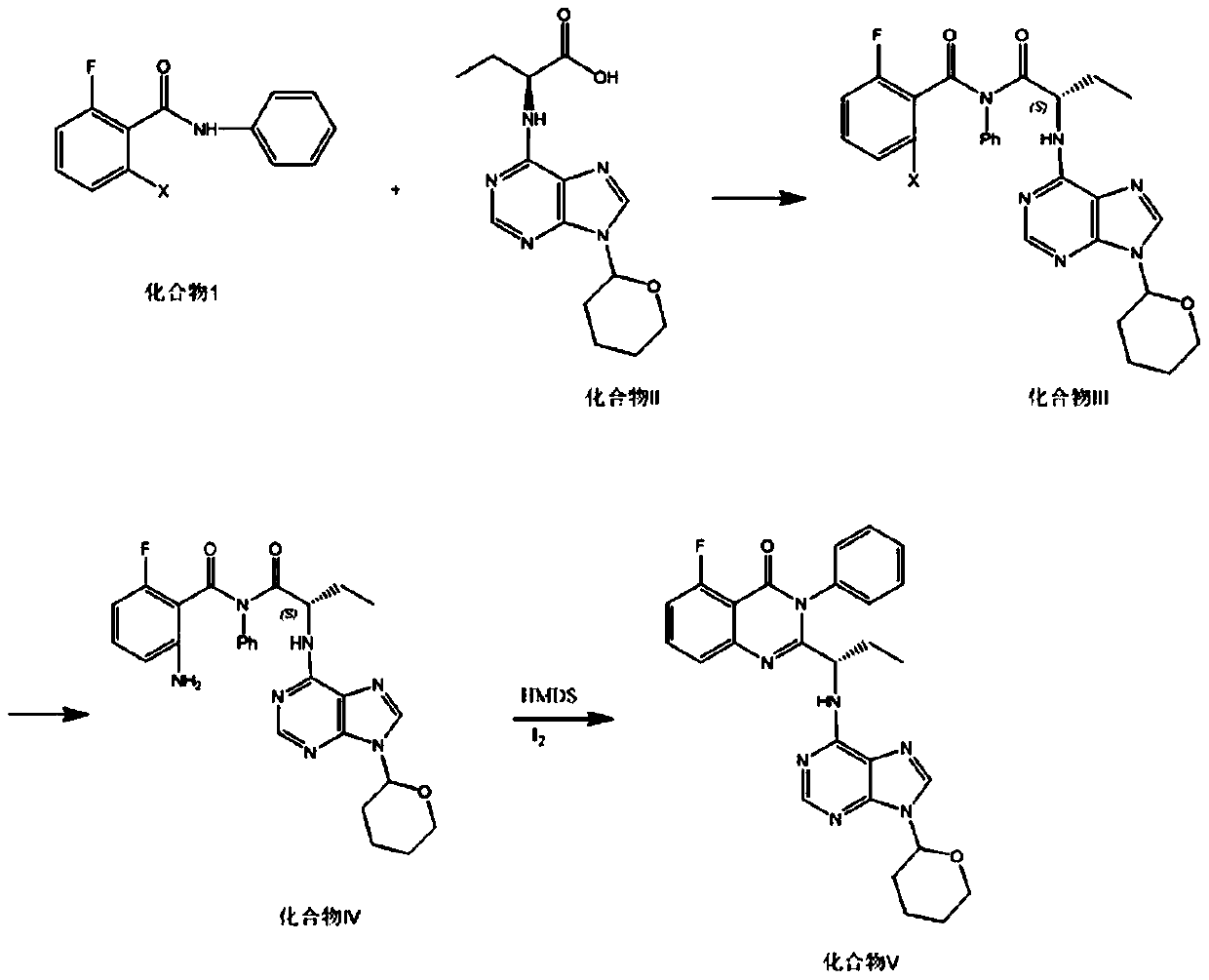
A kind of preparation method of idelaris and its intermediate
A technology of idelaris and intermediates, applied in the direction of organic chemistry, can solve environmental problems and other problems, achieve the effects of reducing solid waste, improving safety and product yield, and avoiding genotoxic raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0037] 3) The preparation of compound V is a ring-closure reaction, and the solvent is dichloromethane, chloroform, tetrahydrofuran, DME (ethylene glycol dimethyl ether), etc., compound IV: solvent (mass ratio) = 1:5 to 1:10. The raw materials for the reaction are compound IV, iodine and HMDS (hexamethylsilazane), and the ratio of the raw materials for the reaction is compound IV: iodine: HMDS=1:0.8:2 to 1:1.2:5; the reaction temperature is 35-80°C. The reaction time is 24 to 48 hours. The reaction product can be purified by isopropanol crystallization.
[0038] 4) Like the method in WO2015095601, hydrochloric acid depyran can be used and sodium bicarbonate can be used to adjust the alkali to obtain the final product Iderarix.
Embodiment 1
[0040] Example 1 Preparation of chlorinated compound III:
[0041] In the flask, equipped with N2 protection, equipped with a thermometer, constant pressure dropping funnel and magnetic stirring. At room temperature, add 8 grams of phosphorus oxychloride to the reaction flask, and slowly add 30 g of triethylamine dropwise. Maintain the temperature at 5-40°C. After the drop is completed, react at 30-40°C for 0.5 hours, and add compound I in batches. (X=Cl) 12.5 g, stirred at 20-40°C for 3 to 4 hours. Concentrate under reduced pressure to remove part of the excess hydrogen chloride. Add 30g of DME and stir evenly, add to the constant pressure dropping funnel, set aside.
[0042] In another four-necked flask, install N2 protection, a thermometer and a constant pressure dropping funnel for the above-mentioned acylated materials. Add 15 g of compound II, 12 g of triethylamine, and 60 g of DME. The acylate in the pressure dropping funnel is added dropwise at a controlled temperature ...
Embodiment 2
[0044] Example 2 Preparation of Bromo Compound III:
[0045] In the flask, equipped with N2 protection, equipped with a thermometer, constant pressure dropping funnel and magnetic stirring. At room temperature, add 10 grams of phosphorus oxychloride to the reaction flask, and slowly add 25 g of triethylamine dropwise, maintaining the temperature at 5-40°C, after the dripping, react at 30-40°C for 0.5 hours, and add compound I in batches (X=Br) 15g, stirred at 20-40°C for 3 to 4 hours. Concentrate under reduced pressure to remove part of the excess hydrogen chloride. Add 30g of DME and stir evenly, add to the constant pressure dropping funnel, set aside.
[0046] In another four-necked flask, install N2 protection, a thermometer and a constant pressure dropping funnel for the above-mentioned acylated materials. Add 15.3g of compound II, 10g of triethylamine, and 60g of DME. The acylate in the pressure dropping funnel is added dropwise at a controlled temperature of 5-30°C. Afte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com