Antibody-coupled drug as well as preparation and application thereof
An antibody-conjugated drug and antibody technology, applied in the field of medicine, can solve the problems affecting the pharmacokinetic evaluation and clinical application of ADC drugs, the inability to obtain drug-loading sites to determine ADC products, and the difficulty of ADCs to effectively reach tumor cells, etc. Achieve the effect of clear structure of drug loading site, increase effective drug delivery rate, and increase tumor cell penetration rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
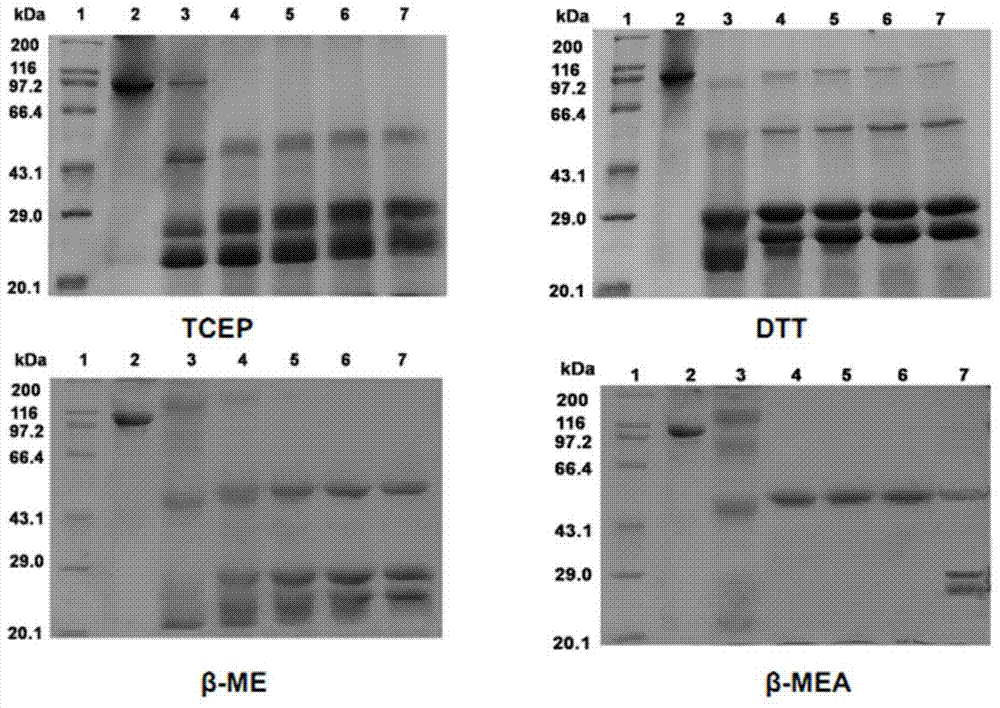
[0068] In this implementation, the F(ab') obtained by pepsin degradation 2 The fragment is reduced with reducing agent β-mercaptoethylamine (β-MEA), the specific method and steps are as follows:
[0069] (1) Degradation of anti-CD20 antibody with pepsin
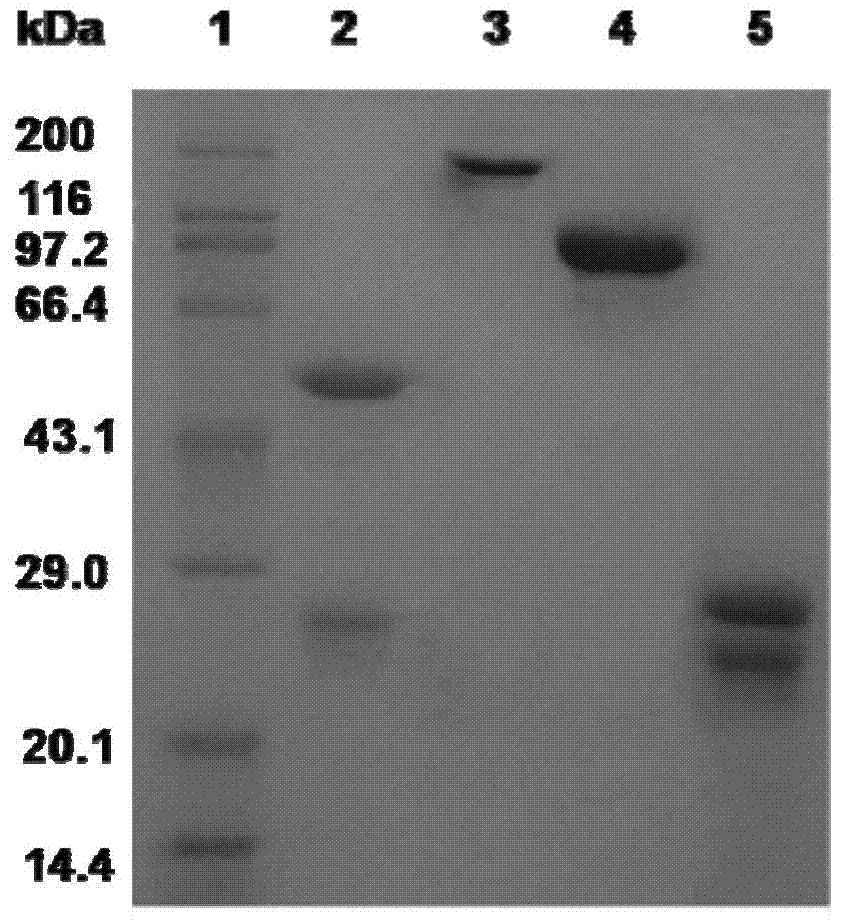
[0070] Weigh 5 mg of anti-CD20 antibody and dissolve it in 2 mL of sodium acetate buffer solution, and measure the concentration value. Add pepsin solution, the mass ratio of anti-CD20 antibody to pepsin is 20:1, react at 37°C for 12h, adjust the pH value to 8.0, stop the reaction, and separate by SDS-PAGE (sodium dodecyl sulfate polyacrylamide Gel electrophoresis) to detect the progress of the reaction and the purity of the sample, such as figure 1 As shown, the results showed that the anti-CD20 antibody was degraded by pepsin to obtain F(ab') 2 fragment.
[0071] (2) Using a reducing agent to convert F(ab') 2 Fragment restore
[0072] F(ab') 2 Fragment concentration is 1.63mg / mL, β-MEA is used as reducing agent, when t...
Embodiment 2
[0078] In this implementation, the F(ab') obtained by pepsin degradation 2 The fragment is reduced with reducing agent β-mercaptoethylamine (β-MEA), the specific method and steps are as follows:
[0079] (1) Degradation of anti-CD20 antibody with pepsin
[0080] Weigh 5 mg of anti-CD20 antibody and dissolve it in 2 mL of sodium acetate buffer solution, and measure the concentration value. Add pepsin solution, the mass ratio of anti-CD20 antibody to pepsin is 20:1, react at 37°C for 12h, adjust the pH value to 8.0, and terminate the reaction to obtain F(ab’) 2 fragment.
[0081] (2) Using a reducing agent to convert F(ab') 2 Fragment restore
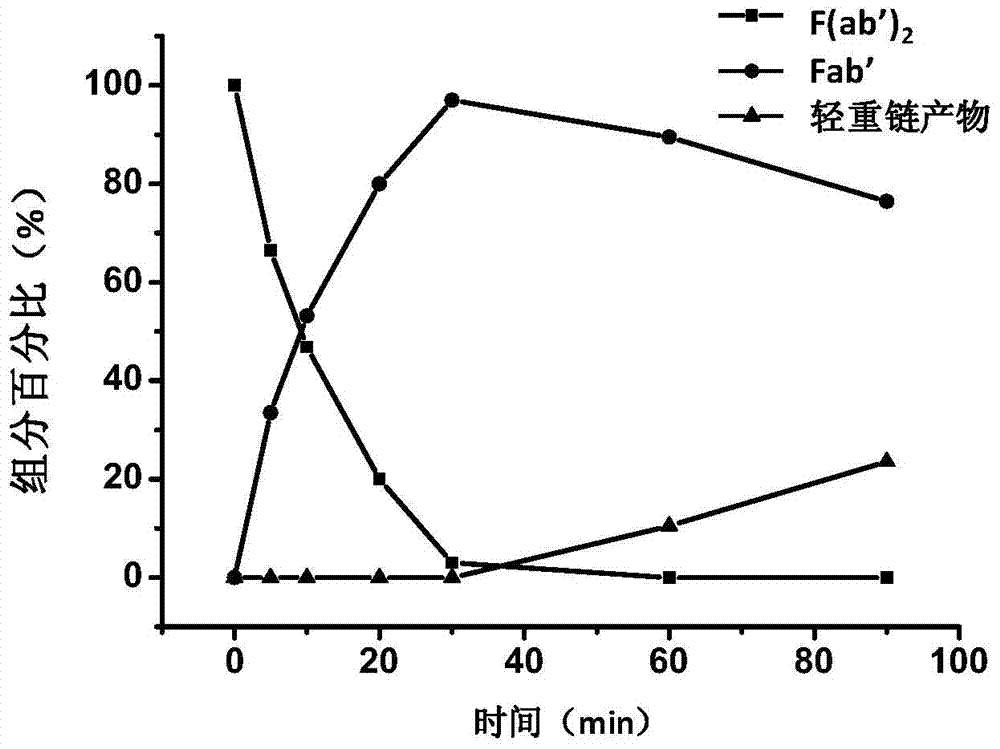
[0082] F(ab') 2 Fragment concentration is 0.5mg / mL, β-MEA is used as reducing agent, when the concentration is 10mM, react at room temperature, start timing when adding reducing agent, respectively at 5, 10, 20, 30, 60, Sampling at 90 minutes, removing the reducing agent through a desalting column, and then using SDS-PAGE to detect ...
Embodiment 3
[0086] In this embodiment, the Fab'-PEG-DOX (doxorubicin) conjugated drug is prepared by the following method, which specifically includes the following steps:
[0087] (1) Degradation of anti-CD20 antibody with pepsin
[0088] Weigh 5 mg of anti-CD20 antibody and dissolve it in 2 mL of sodium acetate buffer solution, and measure the concentration value. Add pepsin solution, the mass ratio of anti-CD20 antibody to pepsin is 20:1, react at 37°C for 12h, adjust the pH value to 8.0, terminate the reaction, and obtain F(ab’) 2 fragment.
[0089] (2) Using a reducing agent to convert F(ab') 2 Fragment restore
[0090] Take the F(ab') obtained in step (1) 2 To 2 mL of the solution, 1.54 mg of solid reducing agent β-MEA was added (the concentration of β-MEA was 10 mM), and the reaction was allowed to stand at room temperature for 30 min. After the reaction is completed, the mixture is separated and purified to obtain a Fab' fragment with reduced disulfide bonds in the hinge regi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com