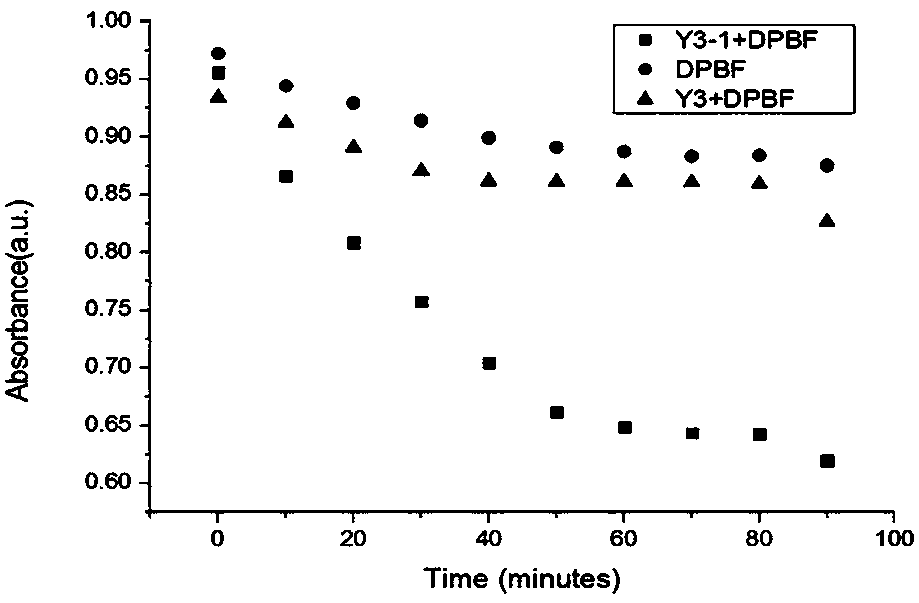
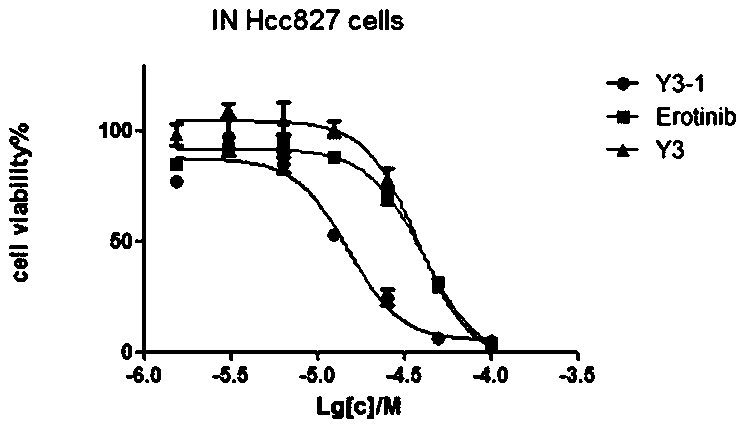
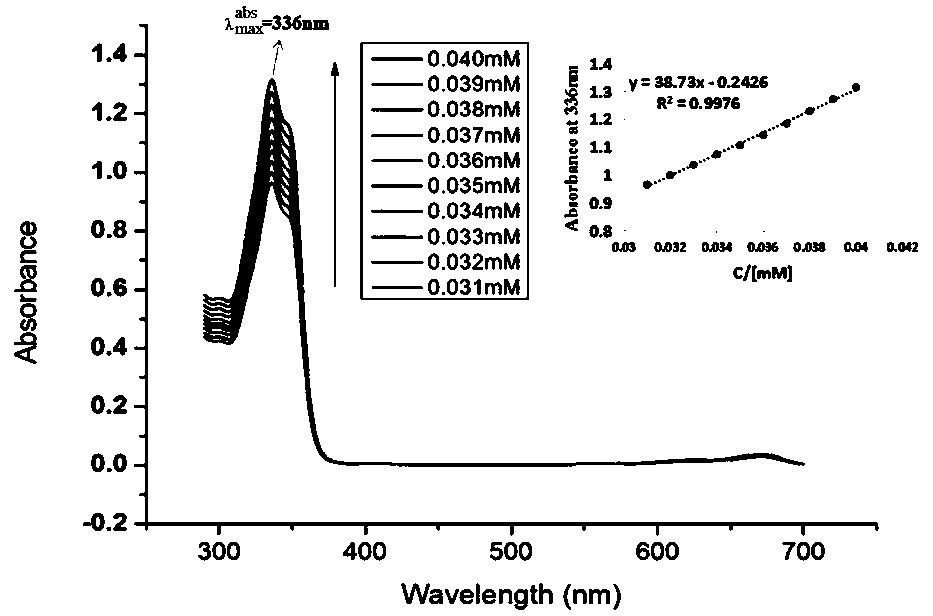
Endoperoxide of controlled release singlet oxygen and preparation method and application thereof
A technology of singlet oxygen and peroxide, applied in organic chemistry, drug combination, antineoplastic drugs, etc., can solve the problems of low conversion rate, endoperoxide decomposition, conversion rate reduction, etc., and achieve high conversion rate and broad The effect of applying the foreground
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Preparation of pyridine ring endoperoxide
[0034] Using 1,4-bis(bromomethyl)benzene as the original raw material, 1-(azidomethyl)-4(bromomethyl)benzene and 1-(4-( Azidomethyl) benzyl) pyridone two intermediates. The 1-(4-(azidomethyl)benzyl)pyridone intermediate is irradiated with laser light, and a 1,4-addition reaction occurs to obtain a compound with an endo-peroxide bridge, that is, a pyridine ring endoperoxide.
[0035] Specific steps are as follows:
[0036] 1) Add 1,4-bis(bromomethyl)benzene (1.218g, 4.614mmol) and sodium azide (0.3019g, 4.644mmol) into a 100mL round bottom flask, then add 10mL to the mixture to remove water The final DMF was stirred at room temperature for 12 hours; then the reaction process was detected with a thin-layer chromatography silica gel plate, and the reaction reached the maximum, and the reaction was stopped, and the DMF was removed by rotary evaporation on a rotary evaporator to obtain a colorless oily crude product, and then add...
Embodiment 2
[0040] The pyridine ring endoperoxide undergoes a click reaction with the small molecule target drug erlotinib to obtain a novel compound of the erlotinib-pyridine ring endoperoxide type.
[0041] Specific steps are as follows:
[0042] Add about 2mL of acetic acid to the Y2-1 (0.2000 g, 0.7346mmol) system to neutralize the remaining methylene blue in the system, add Erlotinib (0.2981g, 0.7577mmol) to the mixed system, add a small amount of copper sulfate pentahydrate CuSO 4 ·5H 2 O and sodium ascorbate are used as catalysts, the solvent is 8mL tetrahydrofuran (THF), 2mL water, 4mL ethanol, THF dissolves organic matter, water dissolves inorganic salt catalyst, tert-butanol is used to mix THF and water to avoid stratification to make it the same system. Set the temperature of the low-temperature reaction bath to 3°C, react for 12 hours, and then point the plate to detect the reaction progress. When the reaction reaches the maximum, stop the reaction, and distill under reduced ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com