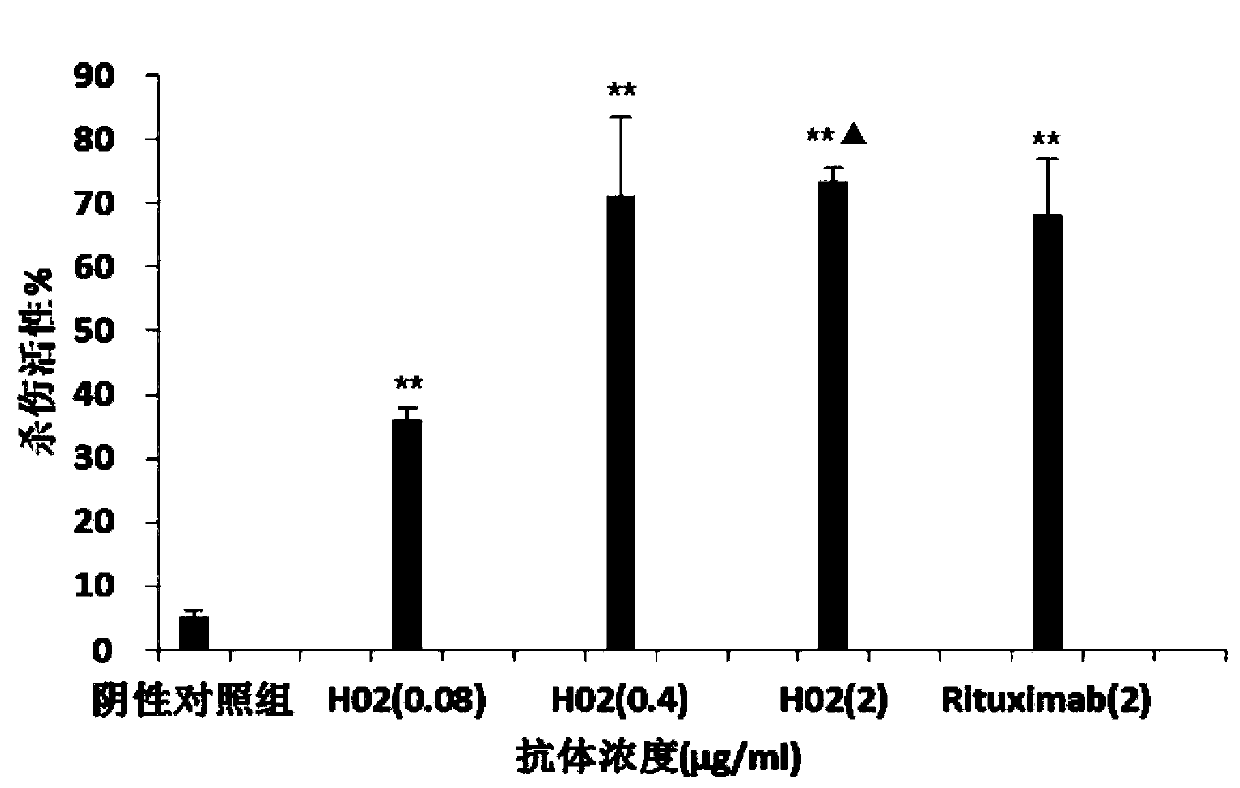
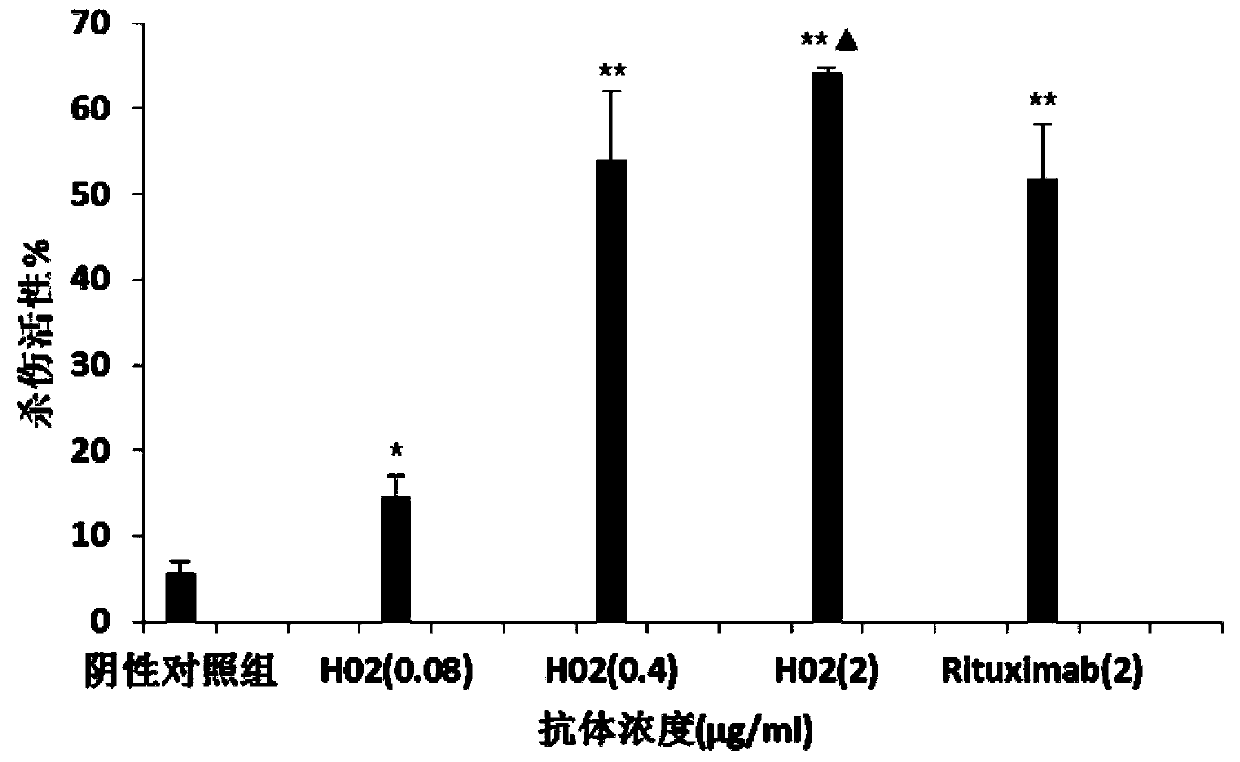
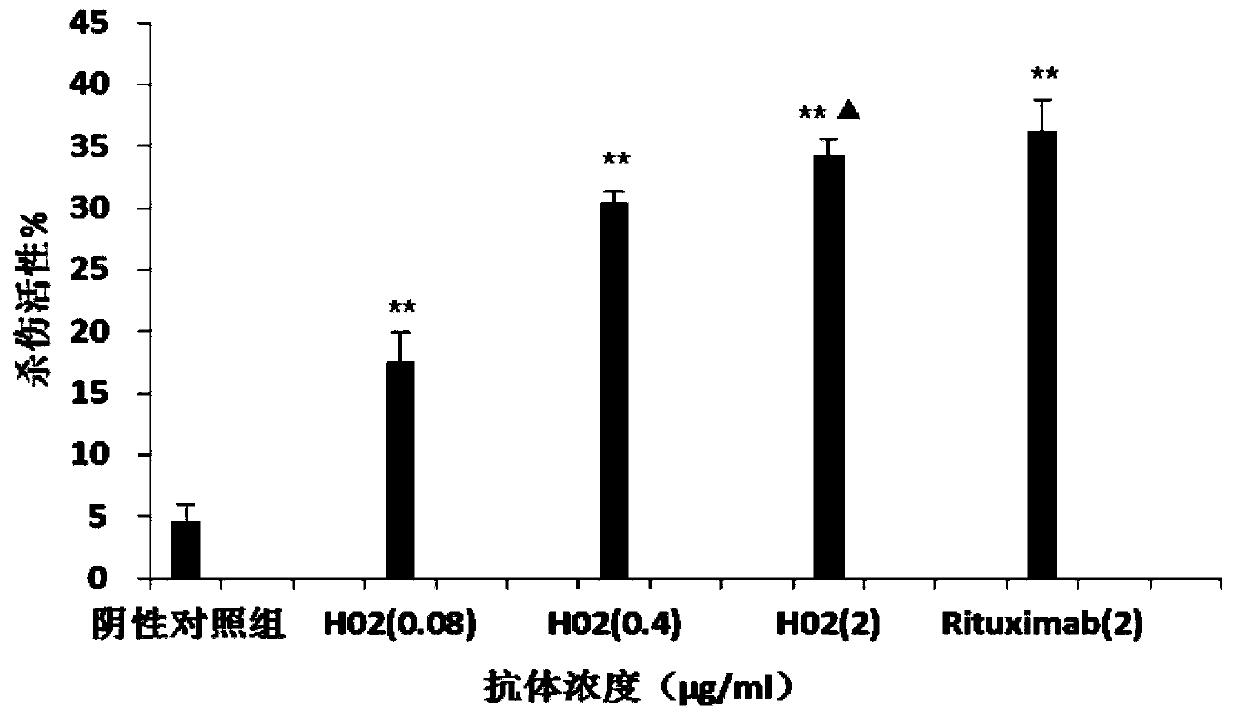
Anti-CD20 monoclonal antibody, preparation method and application thereof
A monoclonal antibody and system technology, applied in the fields of medicine and biotechnology, can solve the problems of host cell toxicity, difficult separation and purification of cell culture expression products, and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0269] The preparation method of the anti-CD20 monoclonal antibody is described in detail below.
[0270] (1) Establish a unique high-efficiency mammalian cell gene expression system and its application
[0271] 1. Experimental research progress
[0272] At present, many scientists have established a variety of inducible expression systems, but they all have their advantages and disadvantages, which requires inventors to select an appropriate expression system according to their own requirements. In general, an ideal inducible expression system needs to meet the following requirements:
[0273] Specificity: The system is not affected by other endogenous factors and can only be activated by exogenous non-toxic drugs.
[0274] Non-interfering: The system components must not interfere with cellular pathways.
[0275] Inducibility: The system has minimal background activity in the inactive state and rapidly produces high levels of gene expression in the activated state.
[027...
Embodiment 1
[0543] Example 1, Construction of CD20 Monoclonal Antibody Expression Vector
[0544] Mammalian cell expression vector pDE was donated by the School of Life Sciences, Tongji University, Shanghai, and the vector uses the DHFR selection marker. The Rituximab heavy chain and light chain gene fragments were excised with HindIII / EcoRI from pUC19 / H02 containing the Rituximab heavy chain and light chain genes confirmed by the sequence, and connected into the expression vector pDE digested with HindIII / EcoRI to construct an expression vector pDE / H02.
Embodiment 2
[0545] Example 2, the source, construction and identification of cell lines for production
[0546] 1. Source and characteristics of host cells
[0547] The host cell is Chinese hamster ovary cell DG44 (Chinese hamster ovary cell DG44, CHO DG44), donated by the School of Life Sciences, Shanghai Jiao Tong University. CD DG44 medium (Cat.NO.12610-010, Invitrogen) was used for routine culture, and the medium was changed for amplification or passage once every 3-4 days.
[0548] 2. Cell Transfection
[0549] The expression vector pDE / H02 was transformed into Escherichia coli DH5α competent cells, and the transformed bacteria were cultured in large quantities, and the plasmids were extracted and purified in large quantities with AxiPrep Plasmid Maxiprep Kit from Axygen Company. The plasmid was linearized by digestion with PvuI, and the concentration of the purified plasmid DNA was adjusted to 1 μg / μl for electroporation to transform CHO DG44 cells.
[0550] On the day of electro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com