Treatment of acute lymphoblastic leukemia
A technology for acute lymphoblastic leukemia, applied in chemical instruments and methods, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, immunoglobulin, etc., can solve the problem of poor efficacy in relapsed acute lymphoblastic leukemia question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
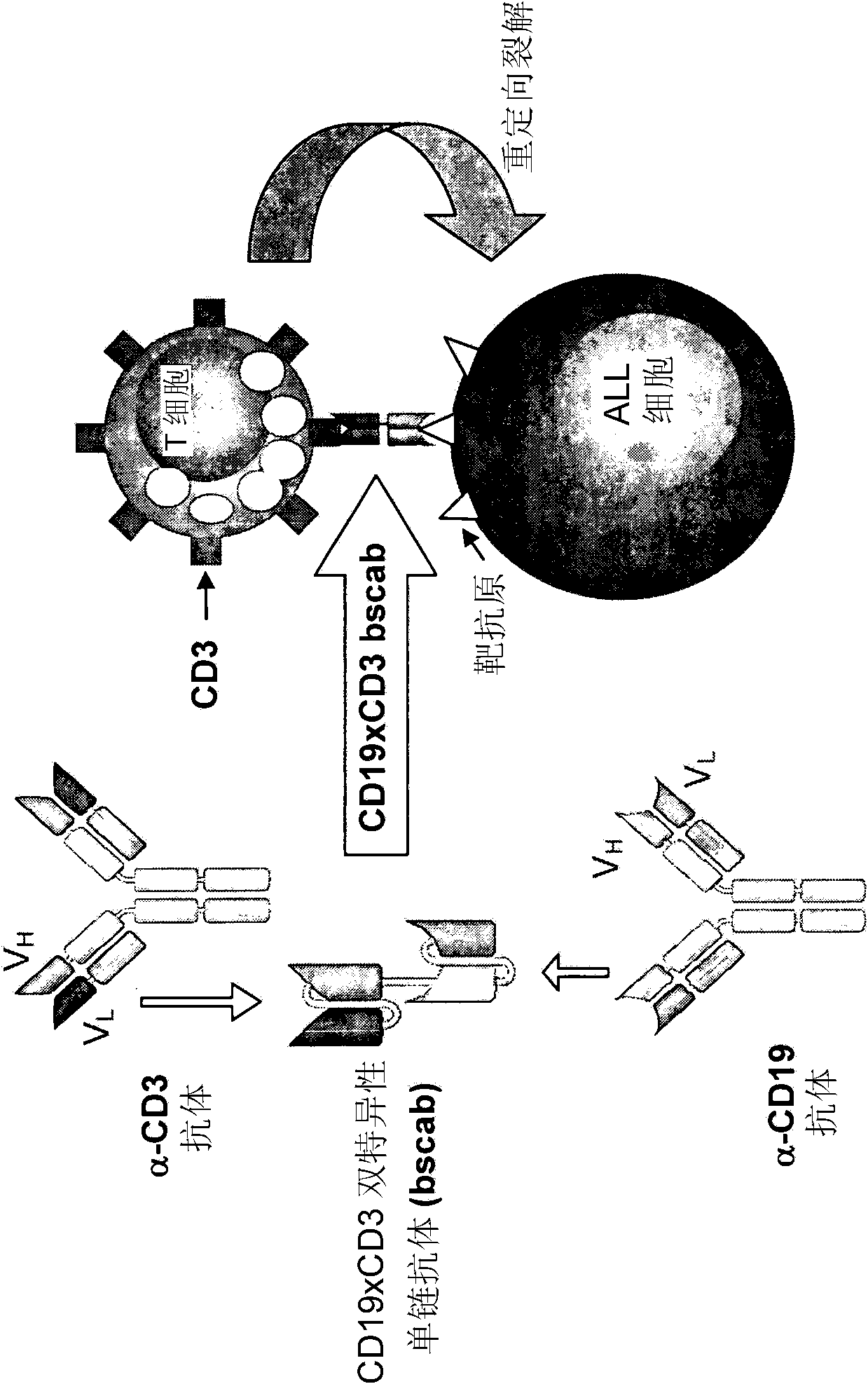
[0117] 1. The production, expression and cytotoxic activity of the CD19xCD3 bispecific single chain antibody are described in WO 99 / 54440. The corresponding amino acid and nucleic acid sequences of the CD19xCD3 bispecific single chain antibody are shown in SED ID NO.1 and 2, respectively. The VH and VL regions of the CD3-binding domain of the CD19xCD3 bispecific single-chain antibody are shown in SED ID NO.7-10, respectively, while the VH and VL regions of the CD19-binding domain of the CD19xCD3 bispecific Shown in SED ID NO.3-6. The corresponding CDR regions are shown in SED ID NO.11-22.
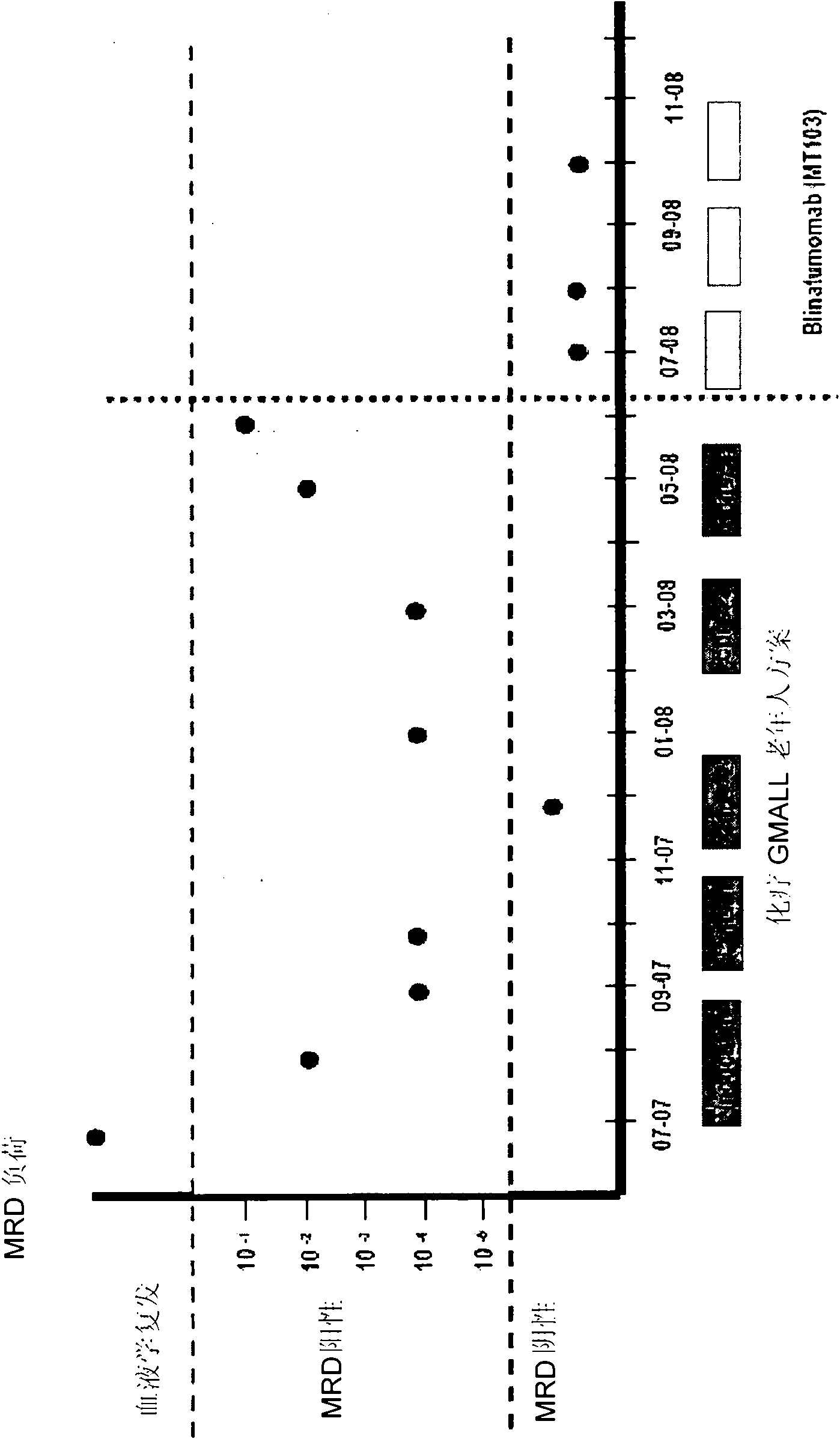
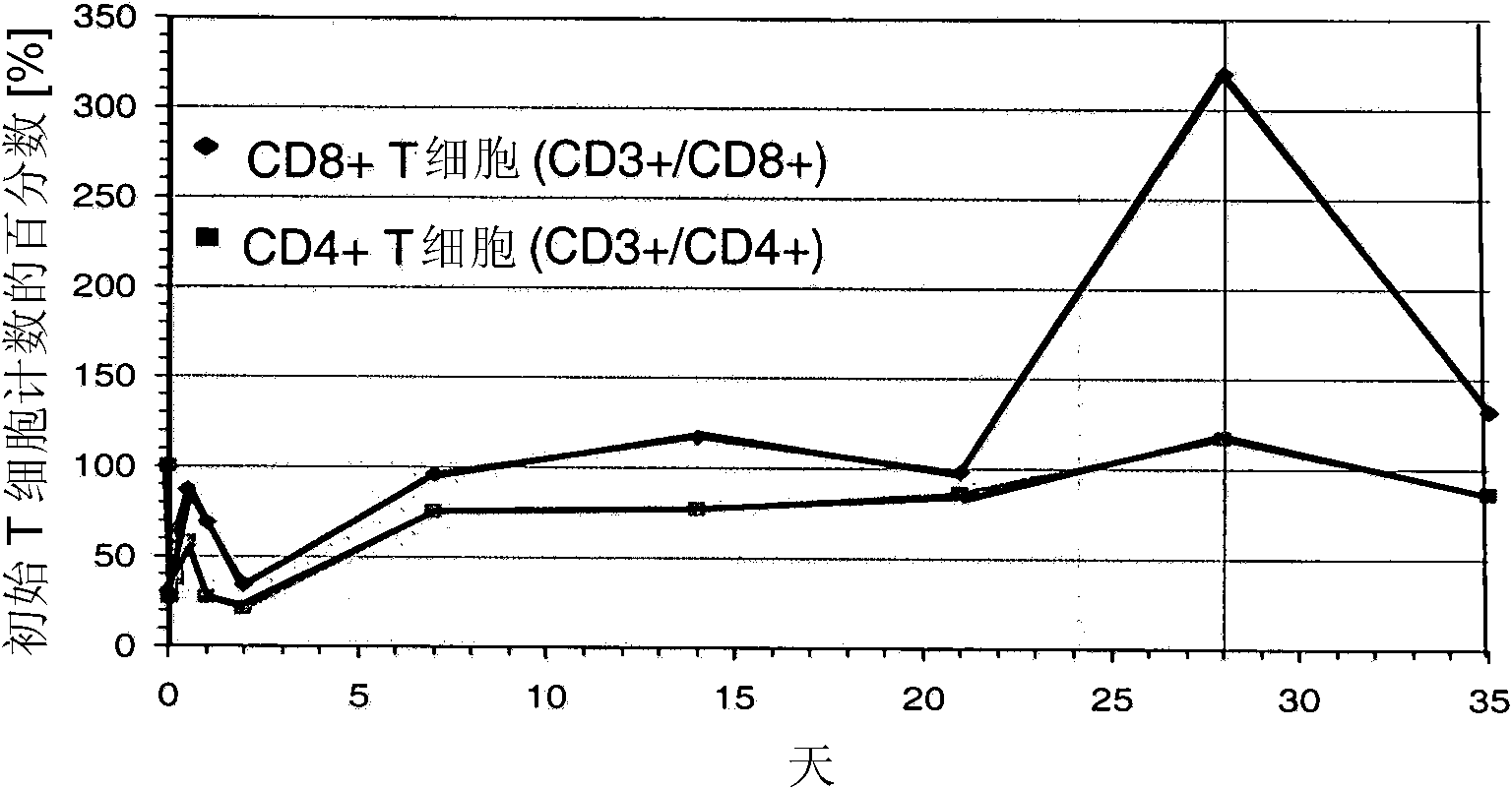
[0118] 2. The ongoing Phase I trial in patients with relapsed B-NHL showed that 60 μg / m 2 CD19xCD3 bispecific single chain antibody per day can obtain high response rate. Response duration was up to 12+ months (in several patients). at 15μg / m 2 Bone marrow infiltrating B-NHL cells were removed every day (Bargou et al., Science 2008).
[0119] 3. Based on these results, a phase II dose...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com