Novel Anti-cd38 antibodies for the treatment of cancer
an anti-cd38 and cancer technology, applied in the introduction of vector-based foreign material, antibody medical ingredients, animal cells, etc., can solve the problem of presence, and achieve the effect of less immunogenicity and improved properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Mouse CD38 Antibodies
[0360]300-19 cells, a pre-B cell line derived from a Balb / c mouse (M. G. Reth et al. 1985, Nature, 317: 353-355), stably expressing a high level of human CD38 were used for immunization of Balb / c VAF mice. Mice were subcutaneously immunized with about 5×106 CD38-expressing 300-19 cells per mouse every 2-3 weeks by standard immunization protocols used at ImmunoGen, Inc. The immunized mice were boosted with another dose of antigen three days before being sacrificed for hybridoma generation. The spleen from the mouse was collected according to standard animal protocols and was ground between two sterile, frosted microscopic slides to obtain a single cell suspension in RPMI-1640 medium. The spleen cells were pelleted, washed, and fused with murine myeloma P3X63Ag8.653 cells (J. F. Kearney et al. 1979, J Immunol, 123: 1548-1550) by using polyethylene glycol-1500 (Roche 783 641). The fused cells were resuspended in RPMI-1640 selection medium containing hypoxanthine-am...
example 2
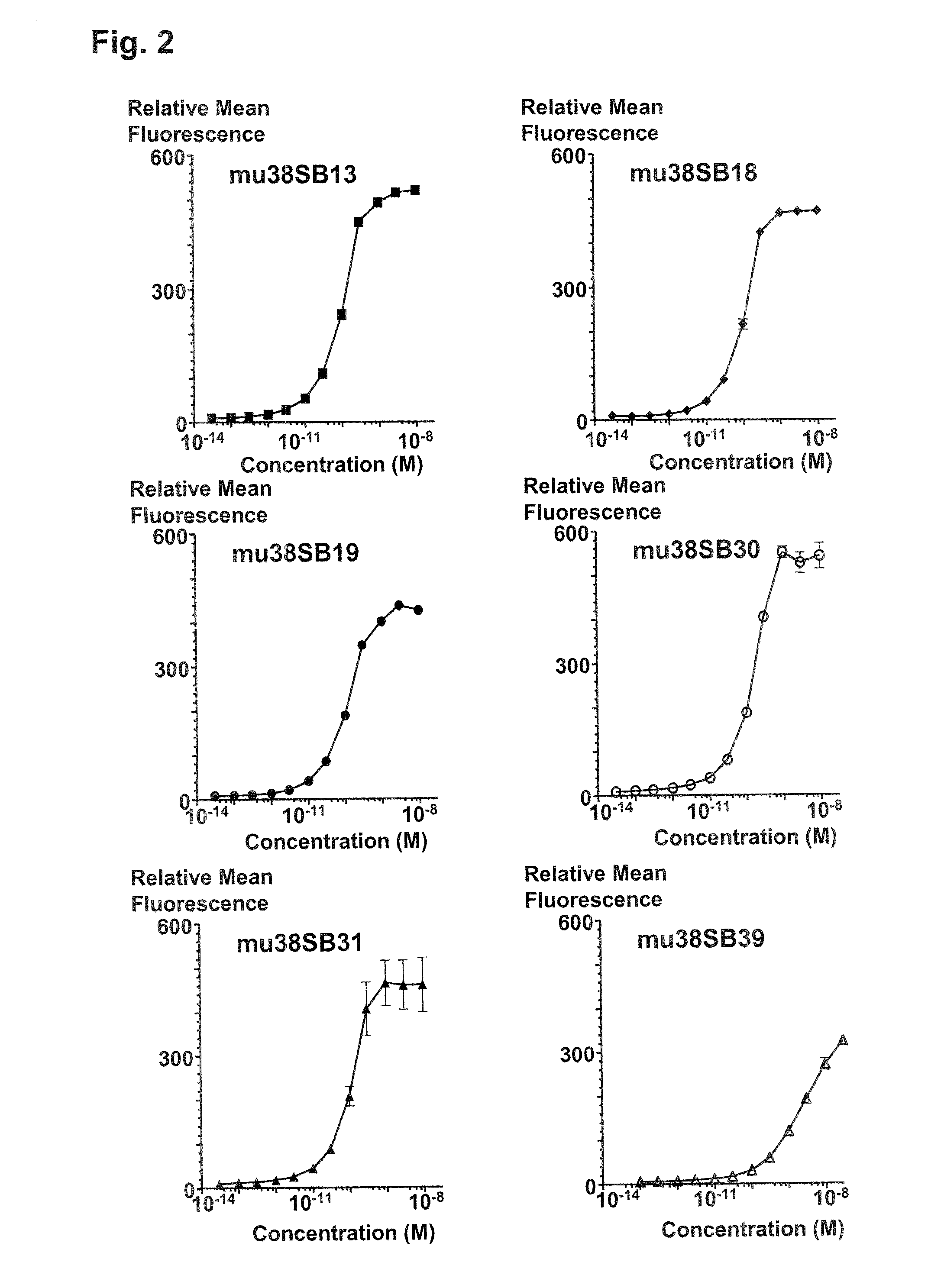
Binding Characterization of Anti-CD38 Antibodies
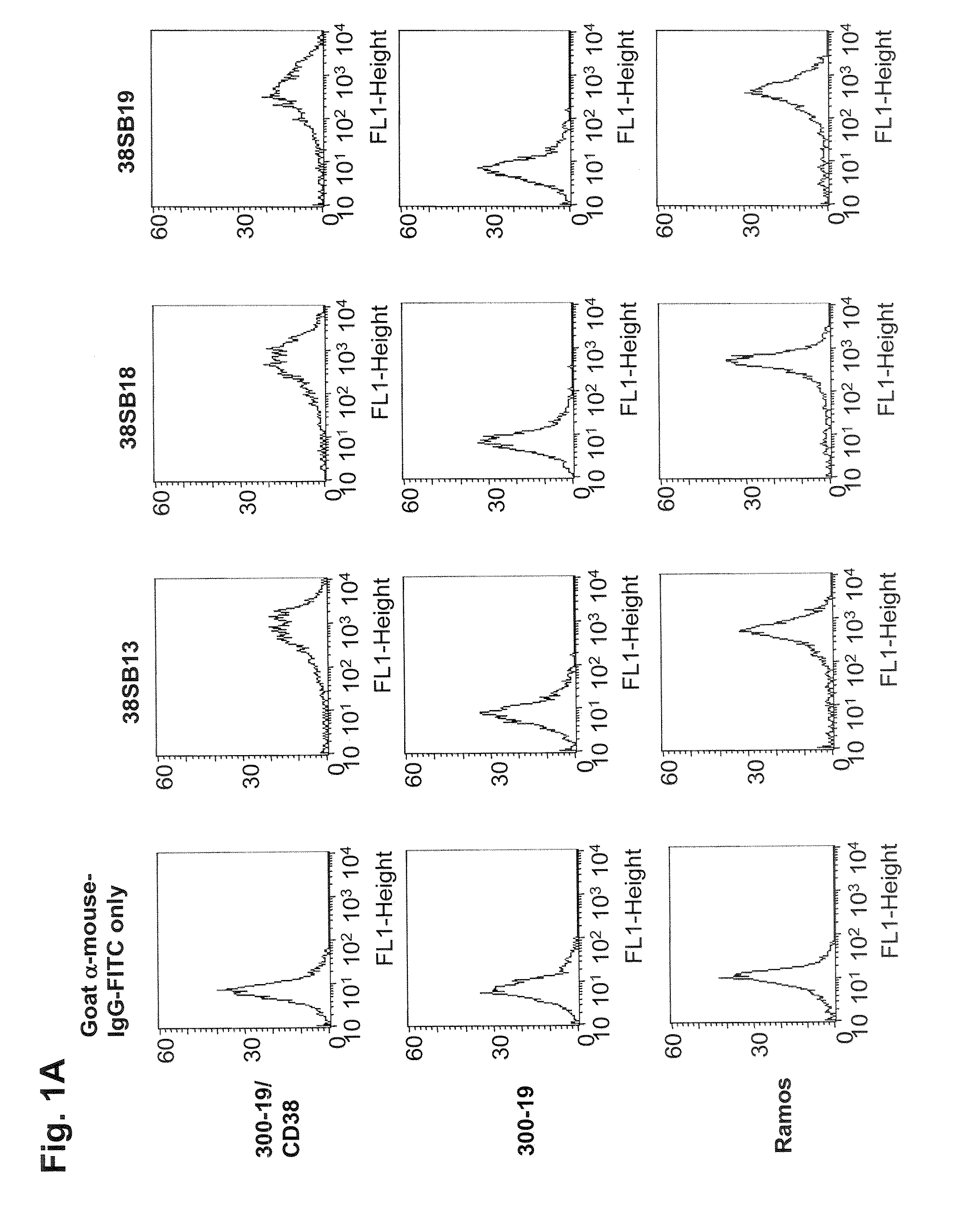
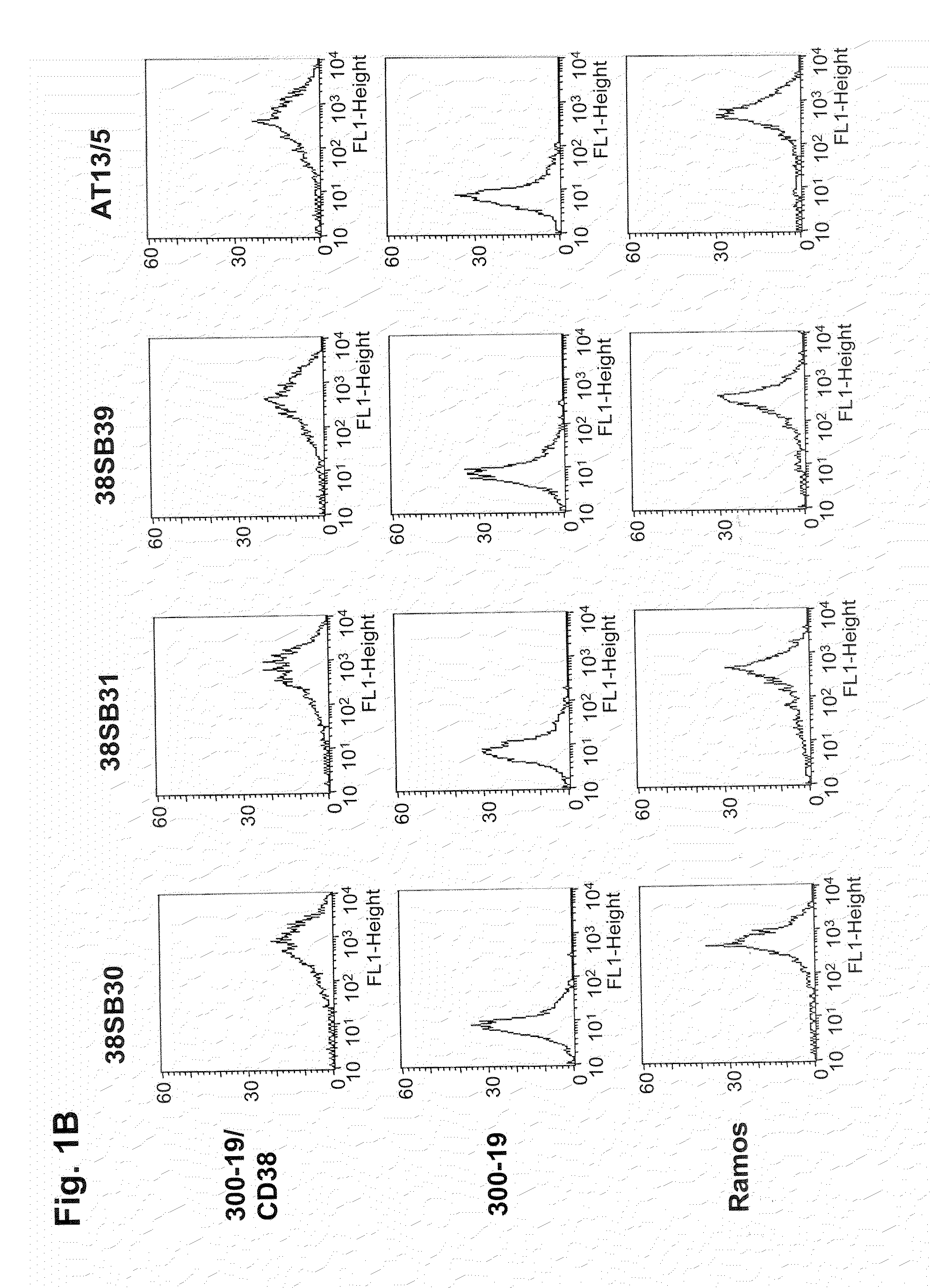
[0362]FACS histograms demonstrating the binding of anti-CD38 antibodies, 38SB13, 38SB18, 38SB19, 38SB30, 38SB31, and 38SB39 to CD38-expressing 300-19 cells and the absence of binding to the parental 300-19 cells are shown in FIG. 1. 38SB13, 38SB18, 38SB19, 38SB30, 38SB31, or 38SB39 antibody (10 nM) was incubated for 3 h with either CD38-expressing 300-19 cells or the parental 300-19 cells (1-2×105 cells per sample) in 100 μL ice-cold RPMI-1640 medium supplemented with 2% normal goat serum. Then, the cells were pelleted, washed, and incubated for 1 h on ice with FITC-conjugated goat anti-mouse IgG-antibody (Jackson Laboratory, 100 μL, 6 μg / mL in cold RPMI-1640 medium supplemented with 2% normal goat serum). The cells were pelleted again, washed, resuspended in 200 μL of PBS containing 1% formaldehyde, and analyzed using a FACSCalibur flow cytometer with CellQuest software (BD Biosciences).
[0363]The FACS histograms of CD38-expressing 300...
example 3
Induction of Apoptosis of Ramos and Daudi Lymphoma Cells, by 38Sb13, 38Sb18, 38SB19, 38SB30, 38SB31, and 38SB39 Antibodies
[0365]The anti-CD38 antibodies, 38SB13, 38SB18, 38SB19, 38SB30, 38SB31, and 38SB39 induced apoptosis of Ramos and Daudi (ATCC CCL-213) lymphoma cell lines and the MOLP-8 multiple myeloma cell line (DSMZ ACC 569). The degree of apoptosis was measured by FACS analysis after staining with FITC conjugates of Annexin V (Biosource PHN1018) and with TO-PRO-3 (Invitrogen T3605). Annexin V binds phosphatidylserine on the outside but not on the inside of the cell membrane bilayer of intact cells. In healthy, normal cells, phosphatidylserine is expressed on the inside of the membrane bilayer, and the transition of phosphatidylserine from the inner to the outer leaflet of the plasma membrane is one of the earliest detectable signals of apoptosis. Binding of Annexin V is thus a signal for the induction of apoptosis. TO-PRO-3 is a monomeric cyanine nucleic acid stain that can ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pharmaceutical composition | aaaaa | aaaaa |
| affinity | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com