Chemokine-4 resisting monoclonal antibody, chemokine-4 resisting hybridoma cell line and applications of monoclonal antibody
A hybridoma cell line, monoclonal antibody technology, applied in the field of hybridoma cell line and application, anti-chemokine 4 monoclonal antibody, can solve the problem that the research is not very clear and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] Preparation of recombinant human anti-chemokine 4 (rhcxcl4-his) and recombinant mouse anti-chemokine 4 (rmcxcl4-his)
[0078] one. gene sequence:
[0079] rhcxcl4-his: NM_002619.3
[0080] rmcxcl4-his: NM_019932.4
[0081] two. Brief introduction of the purification process: resuspension of bacterial cells in bacteriostasis buffer → bacteriostasis → affinity chromatography → denaturation → renaturation → cation exchange chromatography
[0082] three. Purification process:
[0083] a) Purification of rhcxcl4-his:
[0084] Take 20g of fermented cells, add 1× Chinese (his-tag binding buffer) according to the ratio of 10mL bacteriostasis buffer: 1g cells, and suspend the cells on ice;
[0085] Use 500, 700, 800bar high-pressure homogenate to destroy bacteria respectively;
[0086] Centrifuge the bacteriostasis solution at 4°C, 10,000rpm for 30min, take the supernatant and discard the precipitate, keep 1mL of the supernatant sample, and store at 4°C;
[0087] Wash t...
Embodiment 2
[0114] Preparation of anti-human and mouse chemokine 4 (CXCL4) monoclonal antibodies
[0115] 1. Experimental materials
[0116] 1.1 Reagents: RPMI1640 culture medium was purchased from invitrogen, serum was purchased from hyclone, Freund's complete adjuvant and Freund's incomplete adjuvant, HT and HAR were purchased from Sigma, culture plates were Corning products, HRP-labeled goat anti-rat The secondary antibody was purchased from Jackson Company, and both rhcxcl4-his and rmcxcl4-his for immunization were produced in our laboratory.
[0117] 1.2 Cell strains and experimental mice: Human myeloma cell line SP2 / 0 was cultured in suspension in 1640 medium containing 10% FBS, SD rats, 10-12 weeks old, male, purchased from Shanghai Slack Experimental Animal Co., Ltd.
[0118] 2. Experimental method
[0119] 2.1 SD rat immunization: select 3 male SD rats, mix the rhcxcl4-his antigen with an equal volume of Freund's complete adjuvant, and inject it subcutaneously at multiple point...
Embodiment 3
[0122] Identification of Physicochemical Properties of Monoclonal Antibodies
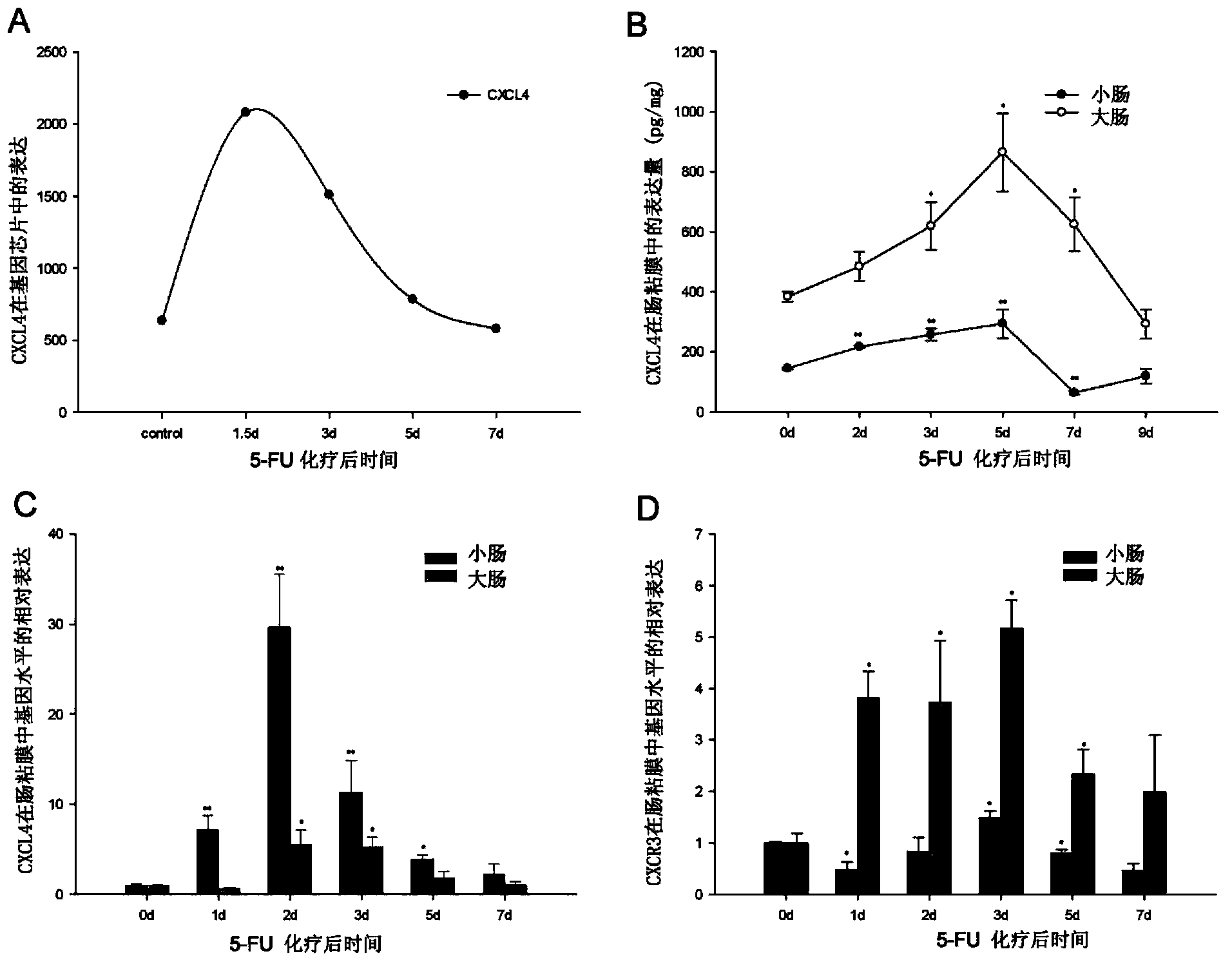
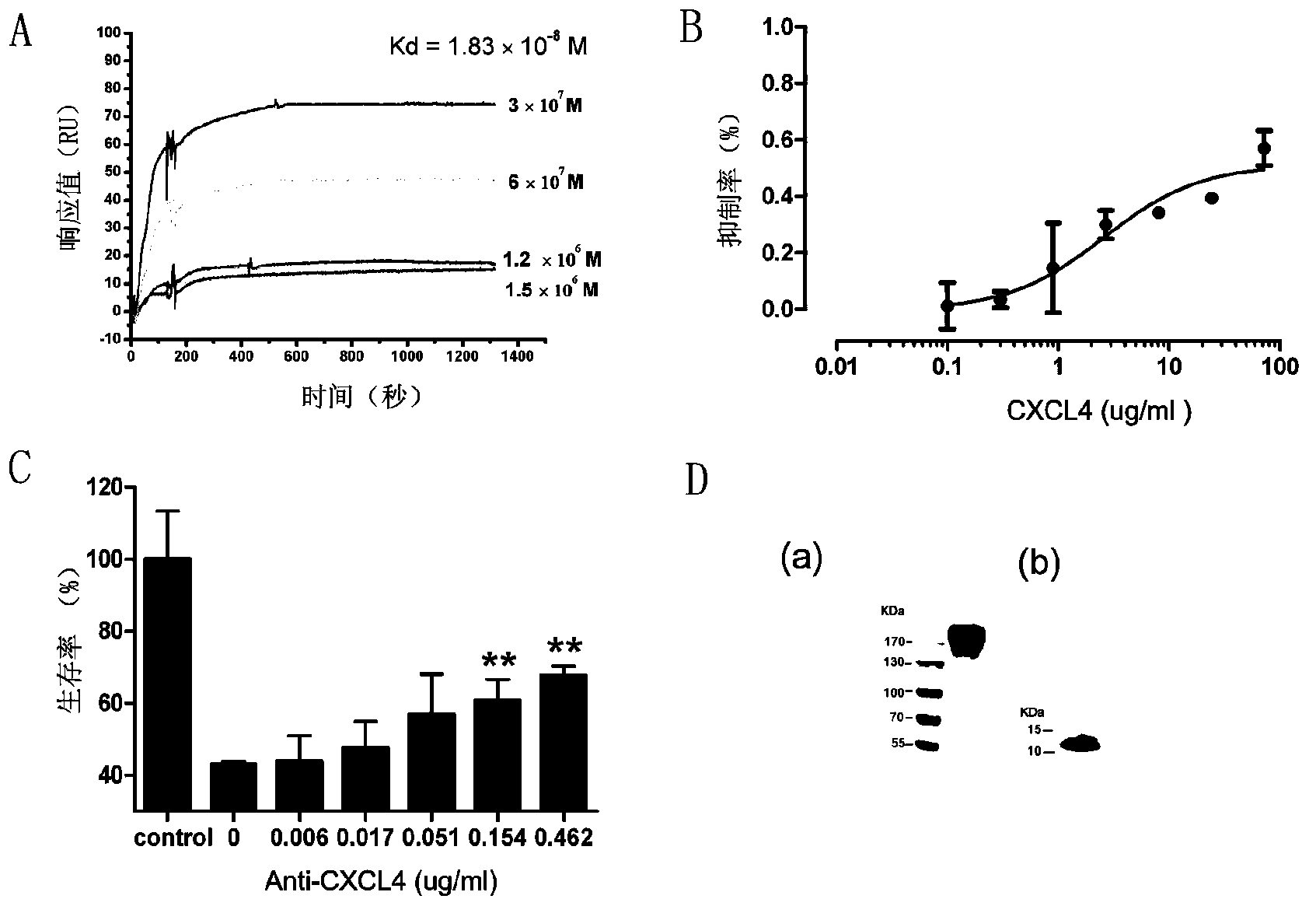
[0123] The invention prepares the Anti-CXCL4 monoclonal antibody capable of neutralizing human and mouse CXCL4 proteins, and performs separation and purification, identification of antibody characteristics, and detection of biological activity. SD rats were immunized with purified recombinant human CXCL4, and a hybridoma cell line secreting anti-recombinant human and mouse CXCL4 monoclonal antibodies was obtained through cell fusion and cloning three times, named 16D6-3. The cell line was continuously cultured in vitro for one month, and the ELISA test proved that the antibody secretion ability was stable. The cell line was injected into nude mice to collect ascites, and the affinity column HiTrap protein G HP (GE Healthcare) was used to purify the antibody in the ascites, and the purity of the antibody as detected by SDS-PAGE reached 98% ( image 3 D-a), and Western blot method was used to detect t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com