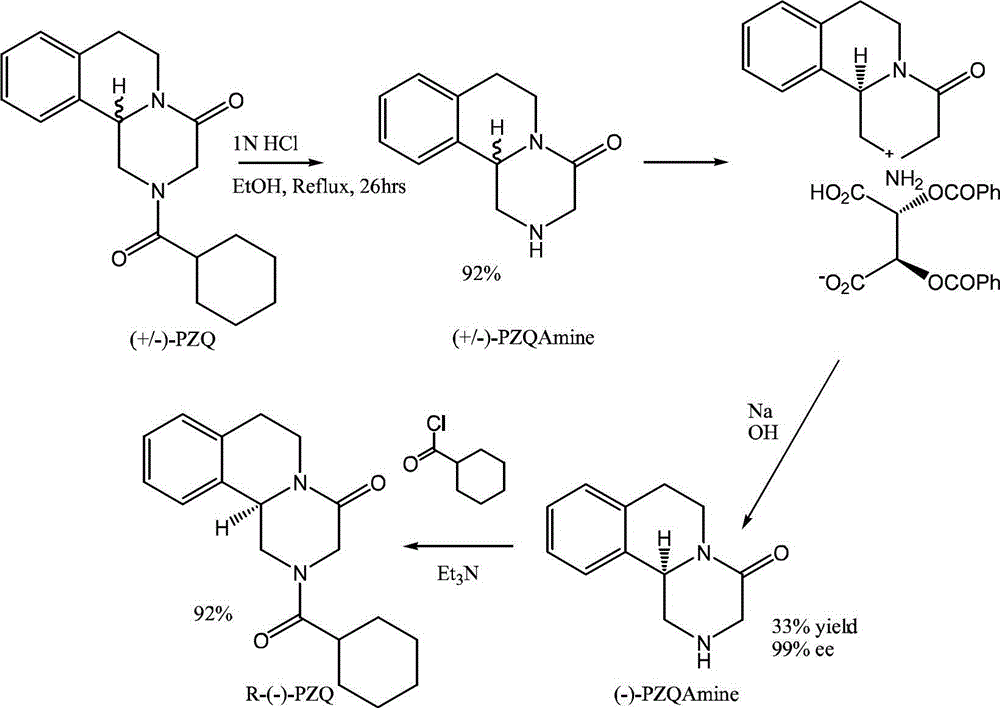
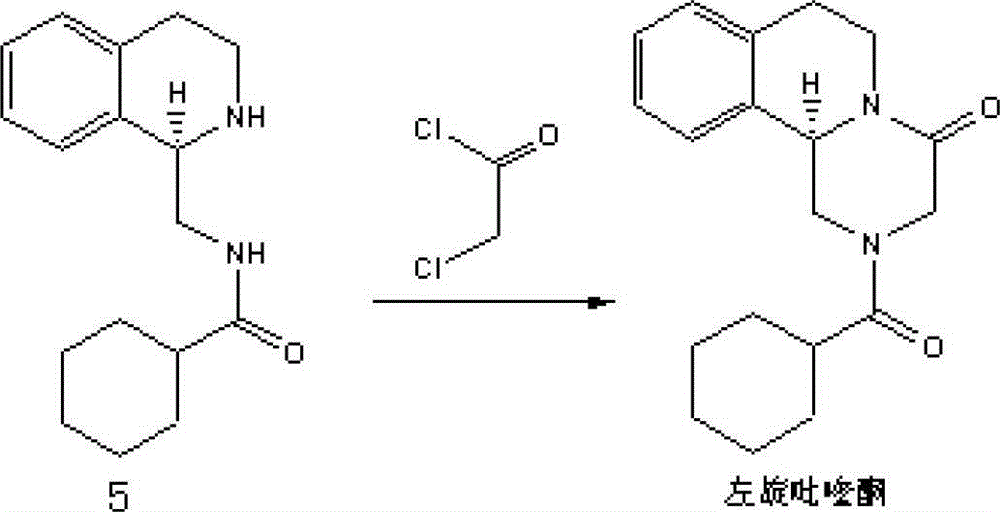
Method for preparing levo-praziquantel
一种左旋吡喹酮、中间体的技术,应用在左旋吡喹酮-praziquantel)的制备领域,能够解决左旋吡喹酮化学合成收率低等问题,达到提升质量标准、成本低、原料易得的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
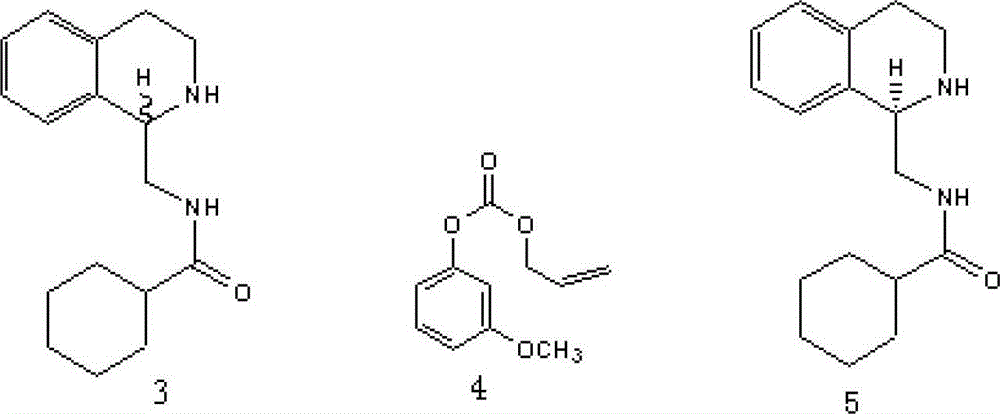
[0024] This embodiment provides a method for preparing intermediate 5 of L-praziquantel:
[0025] Add compound 3 (2.7g, 10mmol), compound 4 (1.35g, 6.56mmol) and toluene (50mL, water content 20mg) into the reactor, after stirring evenly, add 6mg Candida antarctica lipase CAL-A (6000u / g, L3420, Sigma) to start the reaction, the reaction was carried out with stirring at a temperature of 30°C, the reaction process was detected by HPLC, the reaction was stopped after 46-48 hours of reaction, the enzyme was filtered out, washed with 10mL toluene, the organic layer was concentrated under reduced pressure, and the residue was subjected to Silica gel column chromatography (ethyl acetate / n-hexane = 1:15) yielded 1.16 g of intermediate 5 with a yield of about 43%, a melting point of 111-112°C, and an ee value greater than 99%.
[0026] Intermediate 5 NMR data: 1 H NMR (400MHz, CDCl 3 ): δ1.16-1.27 (m, 4H, CH 2 ), 1.35-1.47 (m, 2H, CH 2 ), 1.64-1.85 (m, 4H, CH 2 ), 2.73-2.85 (m, 2H...
Embodiment 2
[0028] This embodiment provides a method for preparing intermediate 5 of L-praziquantel:
[0029] Add compound 3 (1.36g, 5mmol), compound 4 (0.67g, 3.28mmol) and tert-butyl methyl ether (30mL) into the reactor, after stirring evenly, add 12mg Candida antarctica lipase CAL-A (6000u / g , L3420, Sigma) to start the reaction, the reaction was stirred at a temperature of 0°C, the reaction process was detected by HPLC, the reaction was stopped after 66 to 68 hours of reaction, the enzyme was filtered out, washed with 10mL toluene, the organic layer was concentrated under reduced pressure, and the residue was filtered through silica gel. Column chromatography (ethyl acetate / n-hexane = 1:15) yielded 0.52 g of intermediate 5, with a yield of about 38%, a melting point of 112-114°C, and an ee value greater than 93%.
[0030] Intermediate 5 NMR data: the same as in Example 1.
Embodiment 3
[0032] This embodiment provides a method for preparing intermediate 5 of L-praziquantel:
[0033] Add compound 3 (1.36g, 5mmol), compound 4 (0.67g, 3.28mmol) and diethyl ether (30mL) into the reactor, after stirring evenly, add 20mg Candida antarctica lipase CAL-A (6000u / g, L3420 , Sigma) to start the reaction, the reaction was stirred at a temperature of 5°C, the reaction process was detected by HPLC, the reaction was stopped after 46 to 48 hours of reaction, the enzyme was filtered out, washed with 10mL of toluene, the organic layer was concentrated under reduced pressure, and the residue was subjected to silica gel chromatography Column separation (ethyl acetate / n-hexane = 1:15) yielded 0.61 g of intermediate 5 with a yield of about 45%, a melting point of 111-112°C, and an ee value greater than 96%.
[0034] Intermediate 5 NMR data: the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com