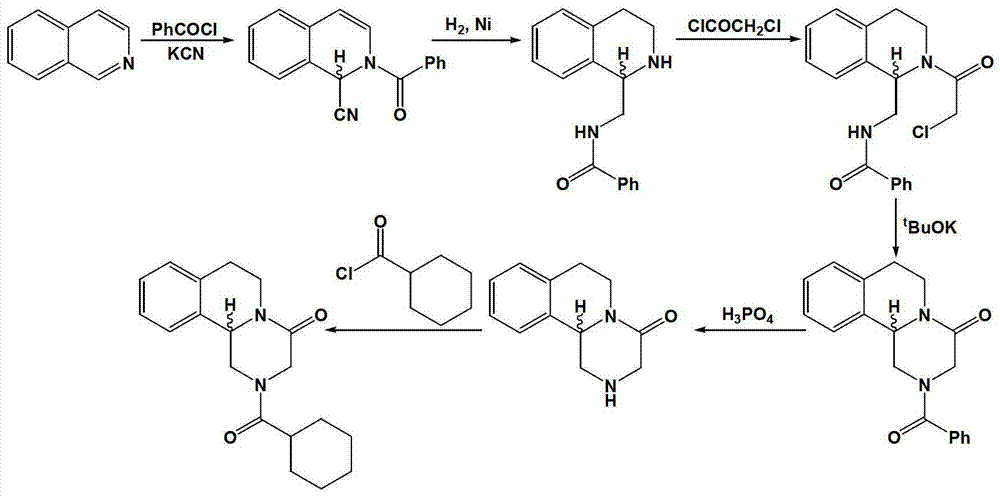
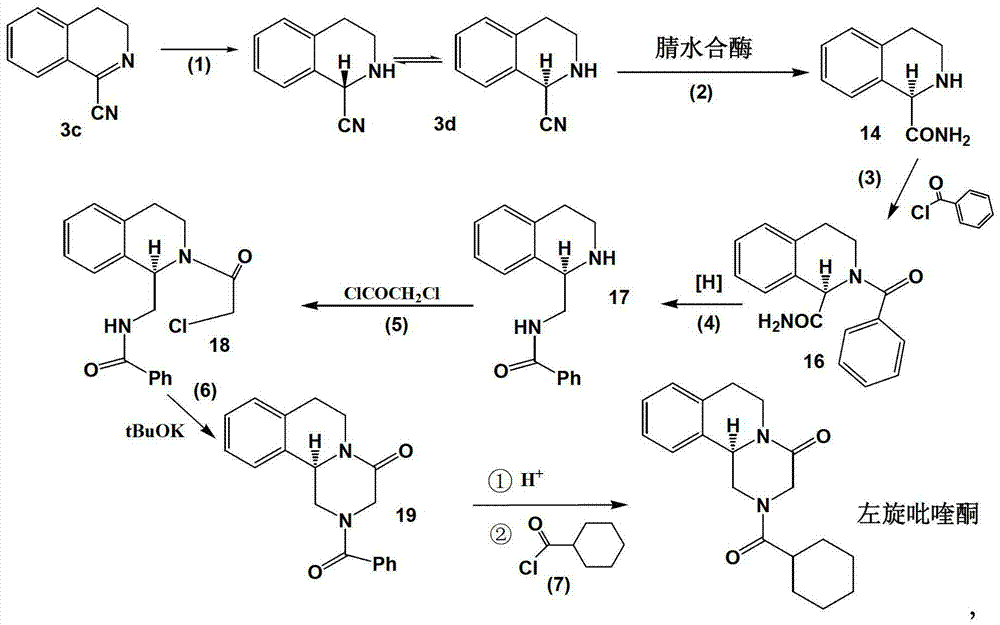
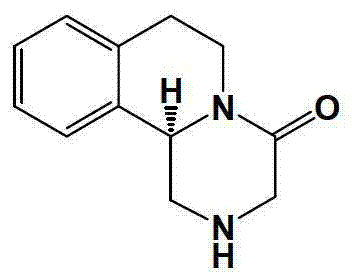
Method of synthetizing levo-praziquantel
A technology of L-praziquantel and a synthesis method, applied in the directions of organic chemistry, fermentation, etc., can solve the problems of low yield of chemical synthesis of L-praziquantel, and achieve the effects of improving quality standards, readily available raw materials, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1 takes following route synthetic tetrahydroisoquinoline carbonitrile
[0030]
[0031] Example 1-1: In a closed container, add compound 3c (78.1g, 0.5mol), ethanol (0.7L) and 10% catalyst Pd / C (6g), after replacing the air in the container with hydrogen, continue to introduce hydrogen ( 1MPa), raised the temperature to 30°C, stirred and reacted for 8 hours, detected that the reaction was complete, recovered the catalyst by filtration, and concentrated the reaction solution under reduced pressure to obtain 74.82g of a solid compound, which was compound 3d (tetrahydroisoquinolinecarbonitrile), with a purity of 97.6 %, yield 94.6%;
[0032] Compound 3d NMR data: 1 H NMR (CDCl 3 ,400MHz,δppm): 3.06–3.10(m,2H,CH 2 ),3.43–3.63(m,2H,CH 2 ), 4.95(s,1H,CH),7.28–7.54(m,4H,ArH), MS(ESI,+ve):m / z:159.1[M+H] + .
example 1-2
[0033] Example 1-2: In a closed container, add compound 3c (78.1g, 0.5mol), methanol (0.7L) and Raney nickel catalyst (12g), replace the air in the container with hydrogen, and continue to introduce hydrogen (1MPa) , stirred and reacted at 25-30 degrees for 8 hours, HPLC detected that the reaction was completely stopped, the catalyst was recovered by filtration, the reaction solution was concentrated under reduced pressure, and the residue was 73.95g of a solid compound, which was compound 3d, with a purity of 94.4% and a yield of 93.5%; It can be directly used in the next reaction without further purification.
[0034] Compound 3d NMR data: 1 H NMR (CDCl 3 ,400MHz,δppm): 3.06–3.10(m,2H,CH 2 ),3.43–3.63(m,2H,CH 2 ), 4.95 (s, 1H, CH), 7.28–7.54 (m, 4H, ArH).
[0035] MS(ESI,+ve):m / z:159.1[M+H] + .
example 1-3
[0036] Example 1-3: Add compound 3c (78.1g, 0.5mol) into 1000mL ethanol, add sodium borohydride (19g, 0.5mol) in batches, stir at 30-40°C for 16 hours, and the system becomes clear. The reaction was completely stopped by HPLC detection, and the organic solvent was distilled off under reduced pressure. The residue was dissolved in dichloromethane, washed with saturated brine, concentrated under reduced pressure to remove the solvent, and obtained 76.0 g of a white solid compound, which was compound 3d, with a yield of 96.1%. The purity is 94.2%.
[0037] Compound 3d NMR data: 1 H NMR (CDCl 3 ,400MHz,δppm): 3.06–3.10(m,2H,CH 2 ),3.43–3.63(m,2H,CH 2 ), 4.95(s,1H,CH),7.28–7.54(m,4H,ArH), MS(ESI,+ve):m / z:159.1[M+H] + .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com