Praziquantel crystal A substance, its preparation method and its applications in medicines and healthcare products
A technology of praziquantel and substance, applied in the field of medicine, can solve the problems such as patents or literature reports of other crystal forms of praziquantel are not found
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] The preparation method of praziquantel crystal type A sample:
[0054] The preparation method of praziquantel crystal type A sample is characterized in that 110 mg of praziquantel sample is completely dissolved at a temperature of 20°C using 20ml of chloroform as a solvent, and the solvent is removed under a vacuum pressure condition of 40°C to prepare praziquantel crystal type samples.
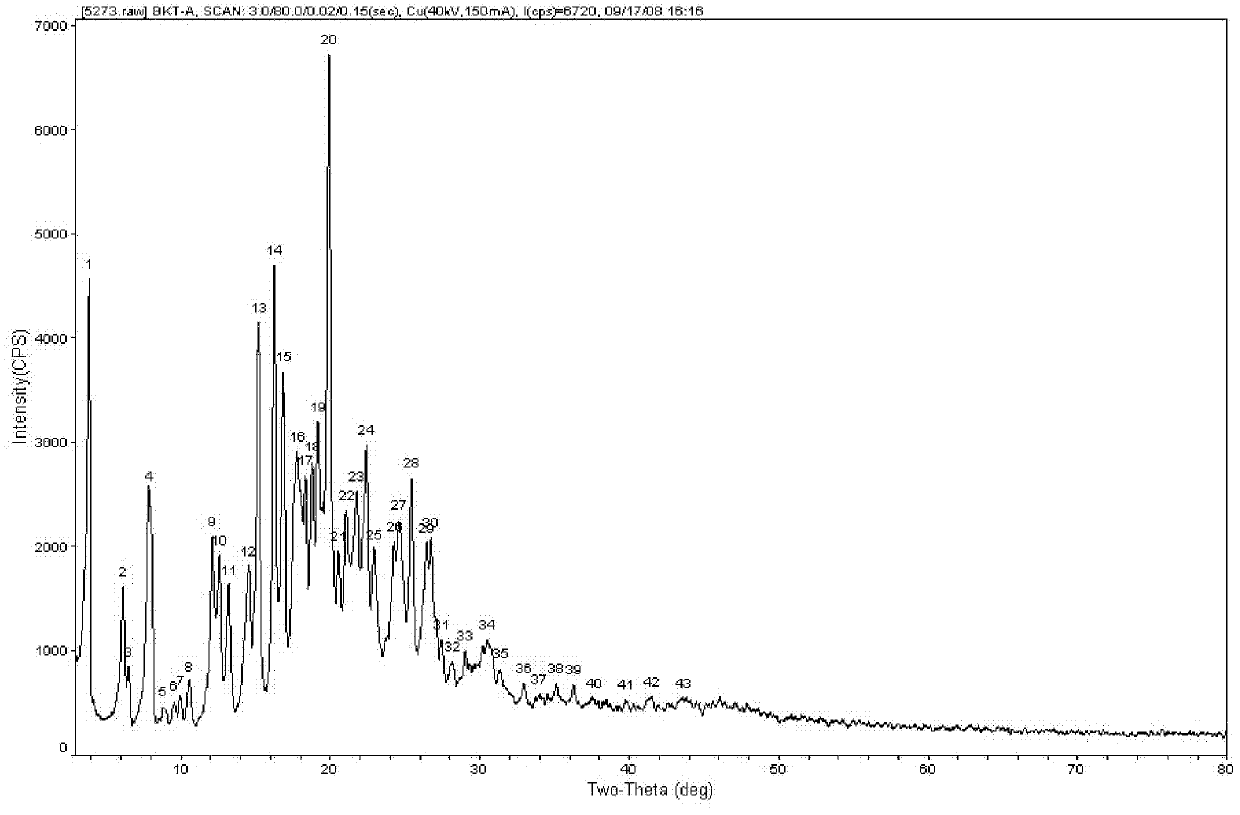
[0055] The powder X-ray diffraction pattern of gained praziquantel crystal form sample is as attached figure 1 shown
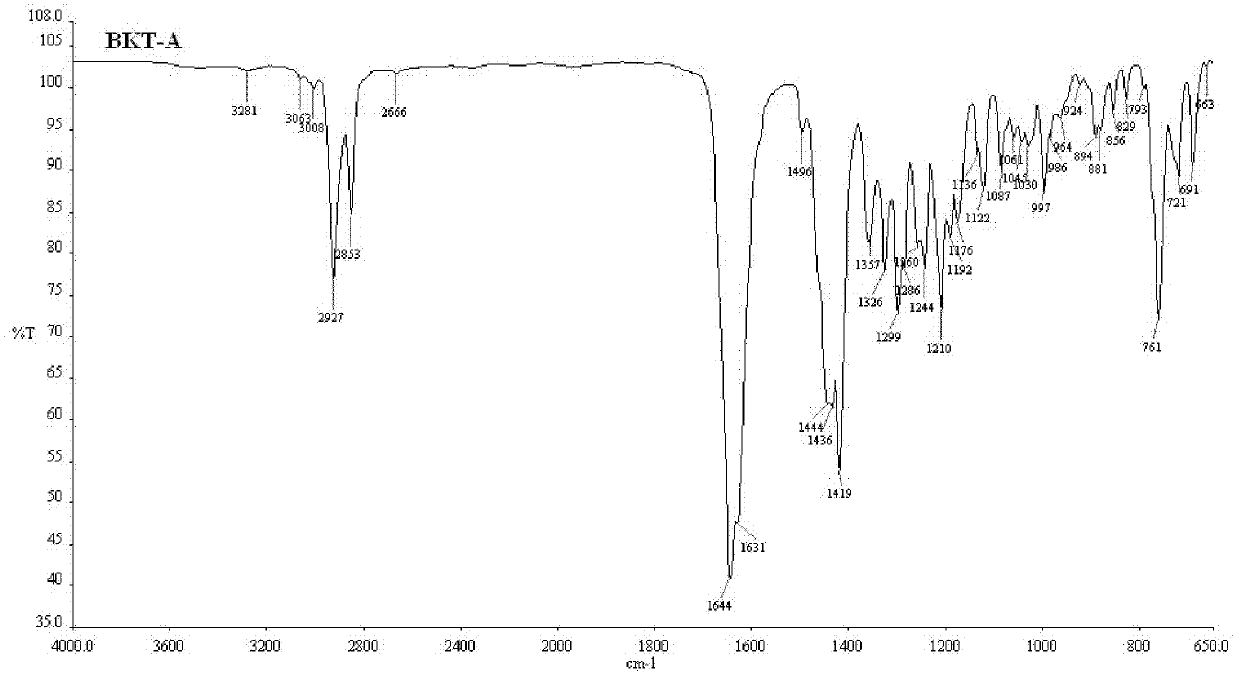
[0056] The infrared absorption spectrum figure of gained praziquantel crystal form sample is as attached figure 2 shown
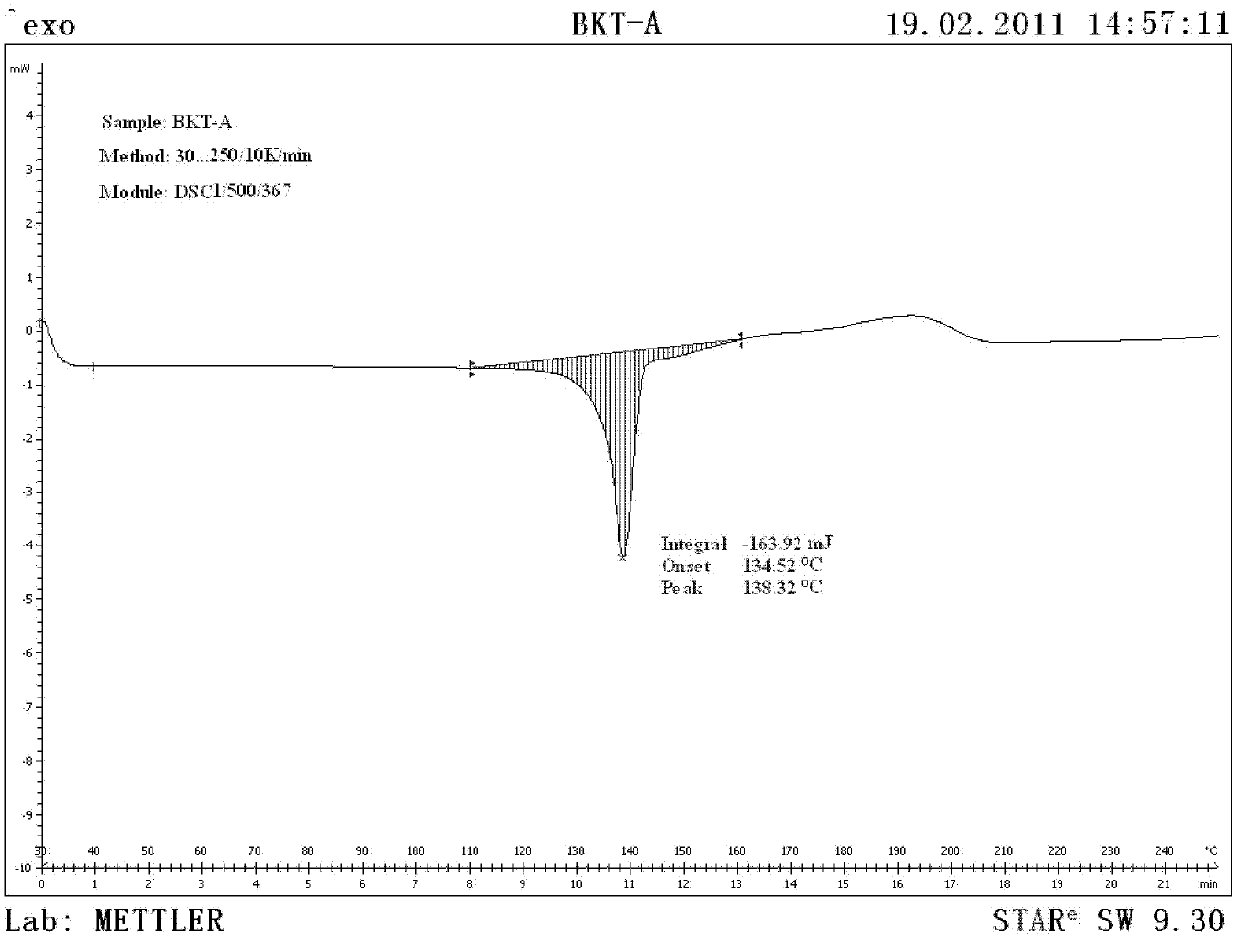
[0057] The DSC collection of illustrative plates of gained praziquantel crystal form sample is as attached image 3 shown
[0058] The results of the above spectrum data show that the crystal form of the crystal obtained in this example is praziquantel crystal type A.
Embodiment 2
[0060] The preparation method of praziquantel crystal type A sample:
[0061] The preparation method of praziquantel crystal type A sample is as follows: firstly, 150 mg of praziquantel sample is completely dissolved at 20°C by adding 10ml of methanol to 20ml of water solvent at room temperature, and then the solvent is removed under vacuum at 40°C to obtain A sample of praziquantel crystal form.
[0062] The powder X-ray diffraction pattern of gained praziquantel crystal form sample is as attached figure 1 shown
[0063] The infrared absorption spectrum figure of gained praziquantel crystal form sample is as attached figure 2 shown
[0064] The DSC collection of illustrative plates of gained praziquantel crystal form sample is as attached image 3 shown
[0065] The results of the above spectrum data show that the crystal form of the crystal obtained in this example is praziquantel crystal type A.
[0066] The problem that needs to be explained: Since there are many ki...
Embodiment 3
[0068] Absorption characteristics and blood concentration characteristics of praziquantel crystal type A samples in rats:
[0069] Wistar rats with a body weight of 205±10g were reared under conventional feeding conditions, free to drink water, and after fasting for 12 hours, 200 mg / kg of praziquantel crystal type A was administered intragastrically, at 0.083, 0.25, and 0.5 before and after administration. , 1, 1.5, 1, 2, 2.5, 3, 4, 6, and 8 hours, about 0.5 ml of blood was collected from the orbit, and centrifuged at 4,000 rpm for 15 minutes to prepare plasma. Take 100μl of plasma, add 1ml of ethyl acetate, vortex for 3min, centrifuge at 13,400rpm for 15min, absorb the upper layer of ethyl acetate, dry it with nitrogen, then dissolve it in 100μl of methanol, inject 20μl for HPLC detection. HPLC detection system: Aligent 1100 high performance liquid chromatography system, chromatographic column: Aligent TC-C18, (150×4.6mm, 5μm), mobile phase: methanol: water = 67:33, injection...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com