Veterinary anti-parasitic preparation containing carnauba wax
An anti-parasitic and brazilian wax technology, applied in the field of veterinary drug preparation preparation, can solve the problem that the clinical effect of the preparation technical scheme is not disclosed or clearly discussed, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
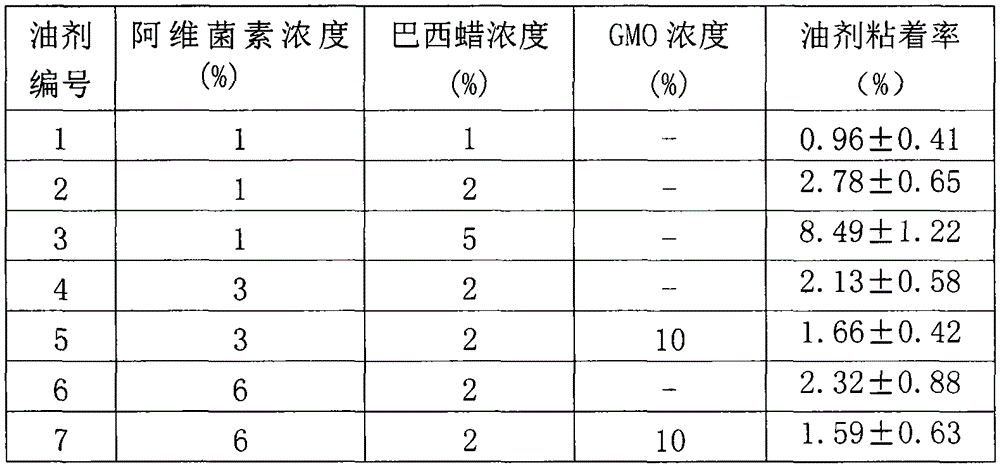
[0019] Embodiment 1, the influence of the presence of GMO in the Brazil wax concentration and preparation on the oil agent adhesion rate (%)
[0020]Oil agent 1 to oil agent 7 in the table take soybean oil as dispersion medium and add to the final volume of oil agent with soybean oil, avermectin concentration (%), carnauba wax concentration (%), GMO concentration (%) in oil agent , are all weight / volume percentage concentrations; The mensuration of oil agent adhesion rate is carried out by the method described in the summary of the invention above, and in the following table, adhesion rate (%) is the average value of 3 parallel samples, and measuring temperature is room temperature (21 -24°C).
[0021]
[0022] The preparation process of above oil agent 1 to oil agent 7 is as follows:
[0023] Mix Brazilian wax and soybean oil, and dissolve Brazilian wax at 85-90°C to obtain a transparent oil solution. After cooling down to room temperature, add abamectin and GMO (oil agen...
Embodiment 2
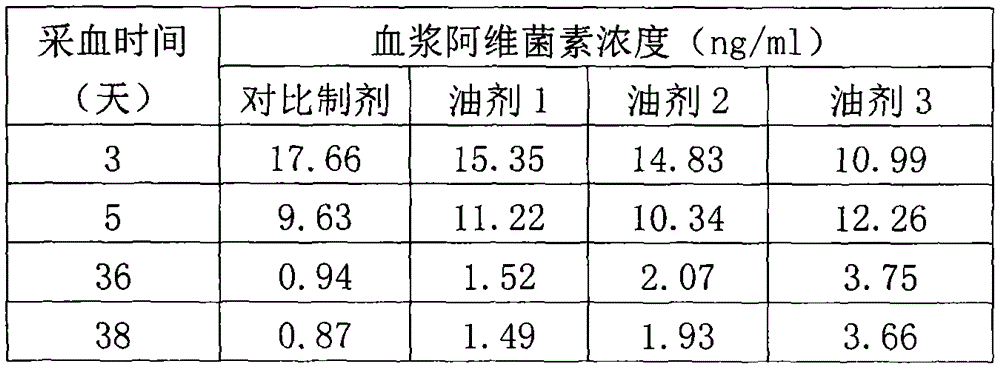
[0025] Example 2: Detection of blood drug concentration after injecting sheep with oil 1, oil 2, oil 3 and comparison preparations in Example 1.
[0026] (1) preparation of contrast preparation: take purity as 96% (B 1a content) of abamectin 5.21g, mixed with 497ml soybean oil, with a high-speed shearing type homogenizer under the condition of 12000-16000r / min, repeated shearing for 1 hour, to obtain a comparison containing 1% abamectin preparation.
[0027] (2) Experimental animals, drug administration and blood sample collection: Select 20 healthy sheep (small-tailed Han sheep) with a body weight of 38-45 kg, and divide them into 4 groups at random, with 5 animals in each group, numbered respectively as group 1, group 2, and group 3. Group 4, group 1 neck subcutaneous injection of contrast preparation 0.4mg / kg b.w., group 2, group 3, group 4 injection of oil 1, oil 2, oil 3 in Example 1 respectively, neck subcutaneous injection , the dosage is 0.4mg / kg b.w. After administ...
Embodiment 3
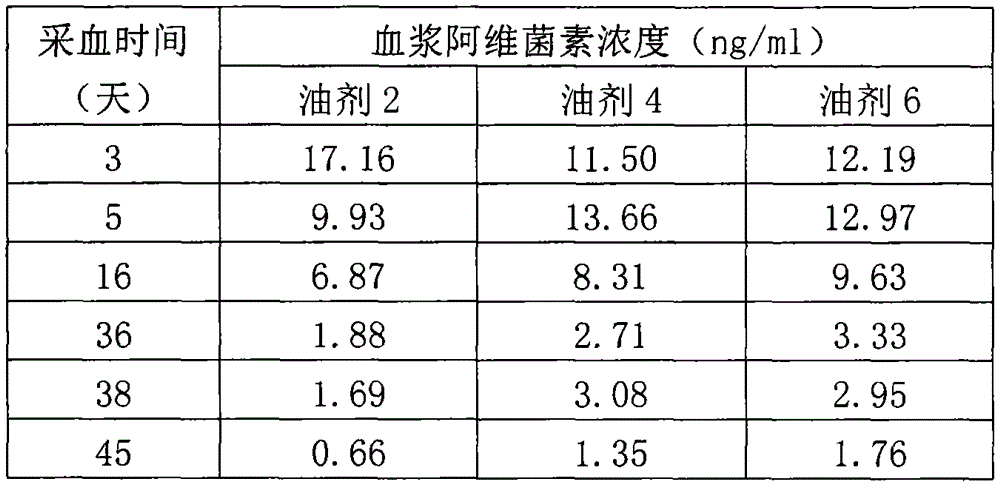
[0033] Embodiment 3, the detection of blood drug concentration after injecting the oil agent 2, the oil agent 4, and the oil agent 6 in the embodiment 1 in the sheep.
[0034] (1) Test animals, drug administration and blood sample collection: select 15 healthy sheep (small-tailed Han sheep) with a body weight of about 40 kilograms, and divide them into 3 groups at random, with 5 animals in each group, and subcutaneously inject the sheep in Example 1 in the neck respectively. Oil 2, oil 4, oil 6, the doses are all 0.4mg / kg b.w. After administration, blood was collected from the jugular vein of sheep on time, accurately 4ml each time, and the blood samples collected at the same time in the same test group were put into the same 20ml centrifuge tube containing heparin sodium, mixed and centrifuged at 3000r / min for 10min, and the upper layer was taken Liquid (plasma), stored at -18 to -20°C.
[0035] (2) Blood sample processing and detection of abamectin in plasma: the method of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com