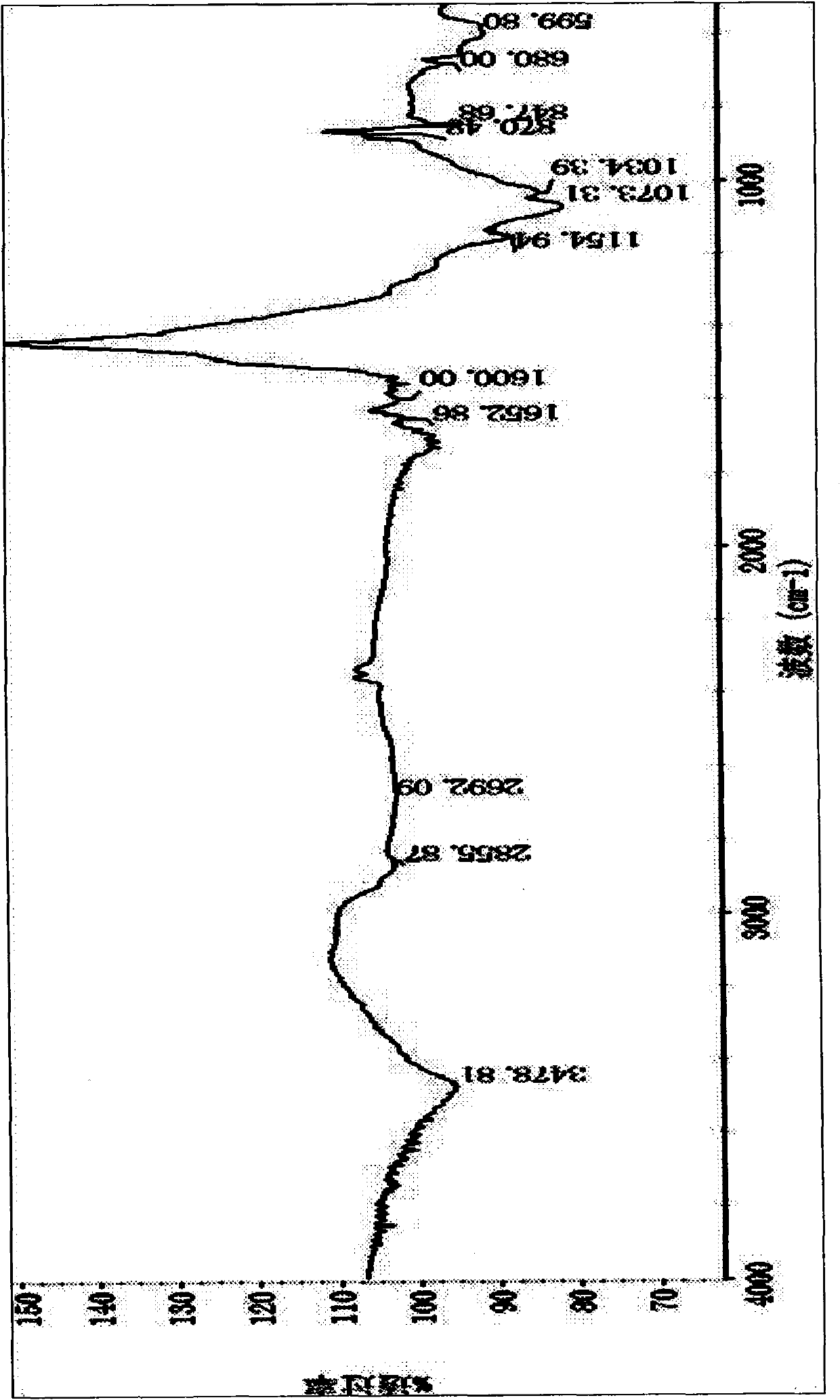
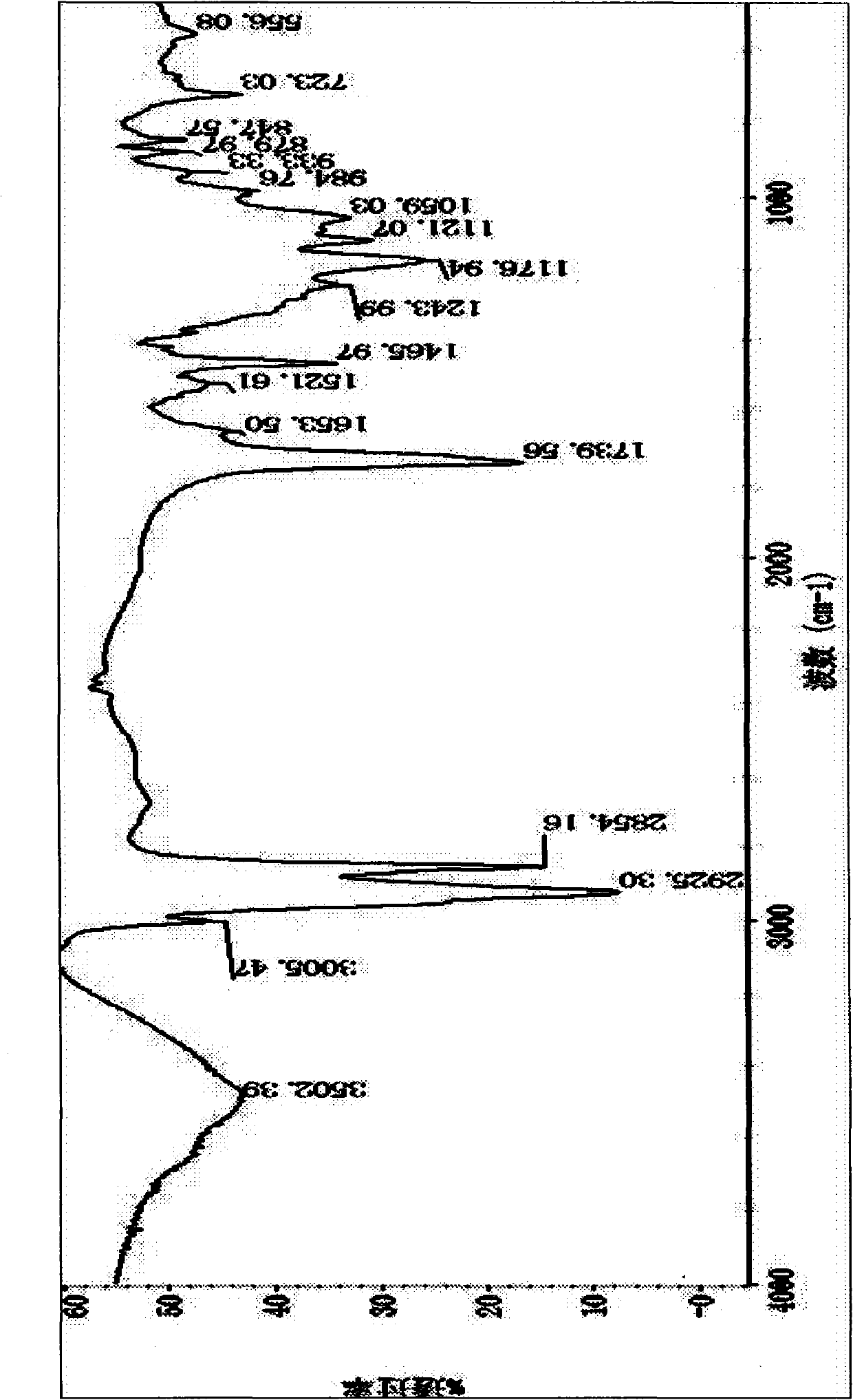
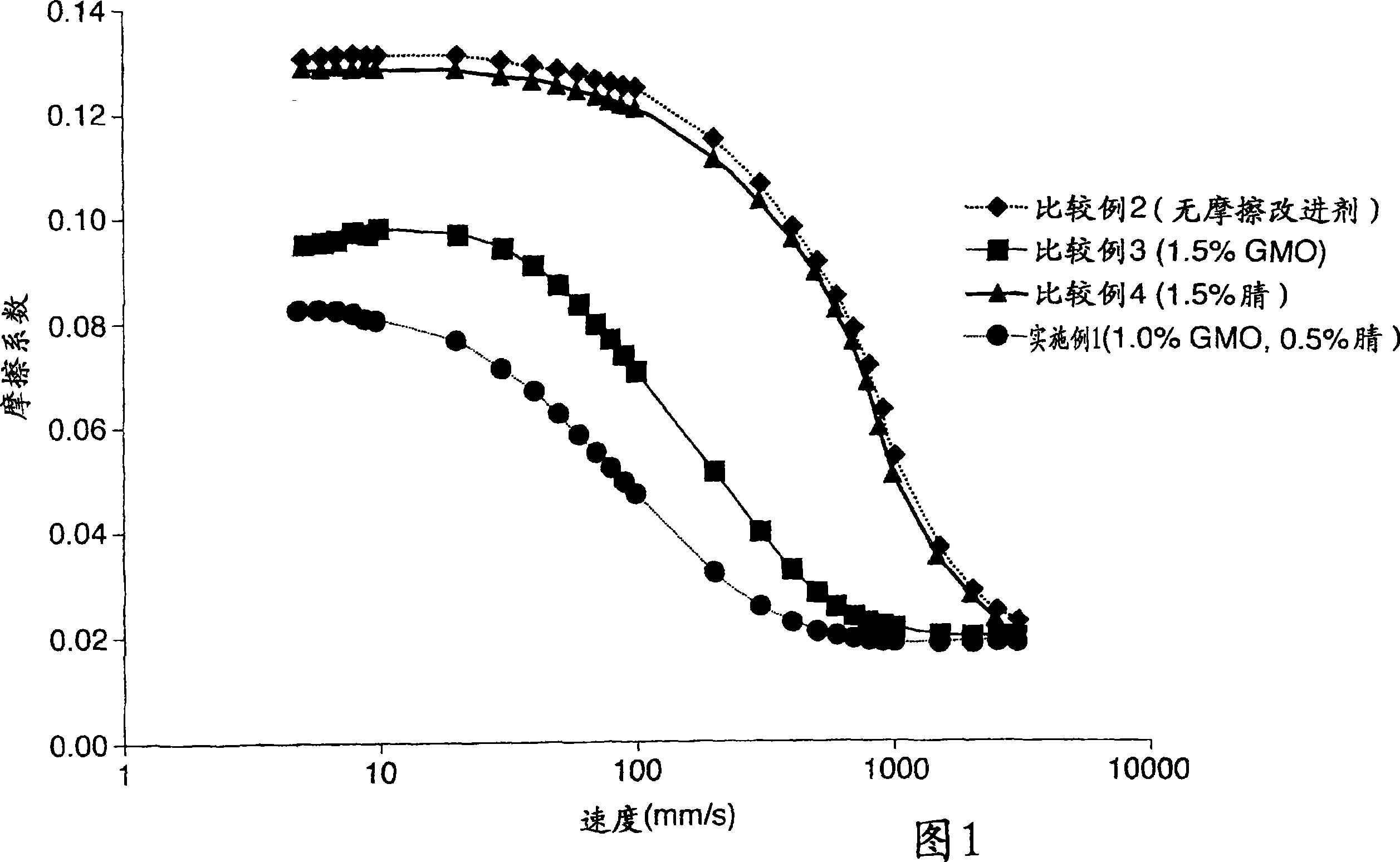
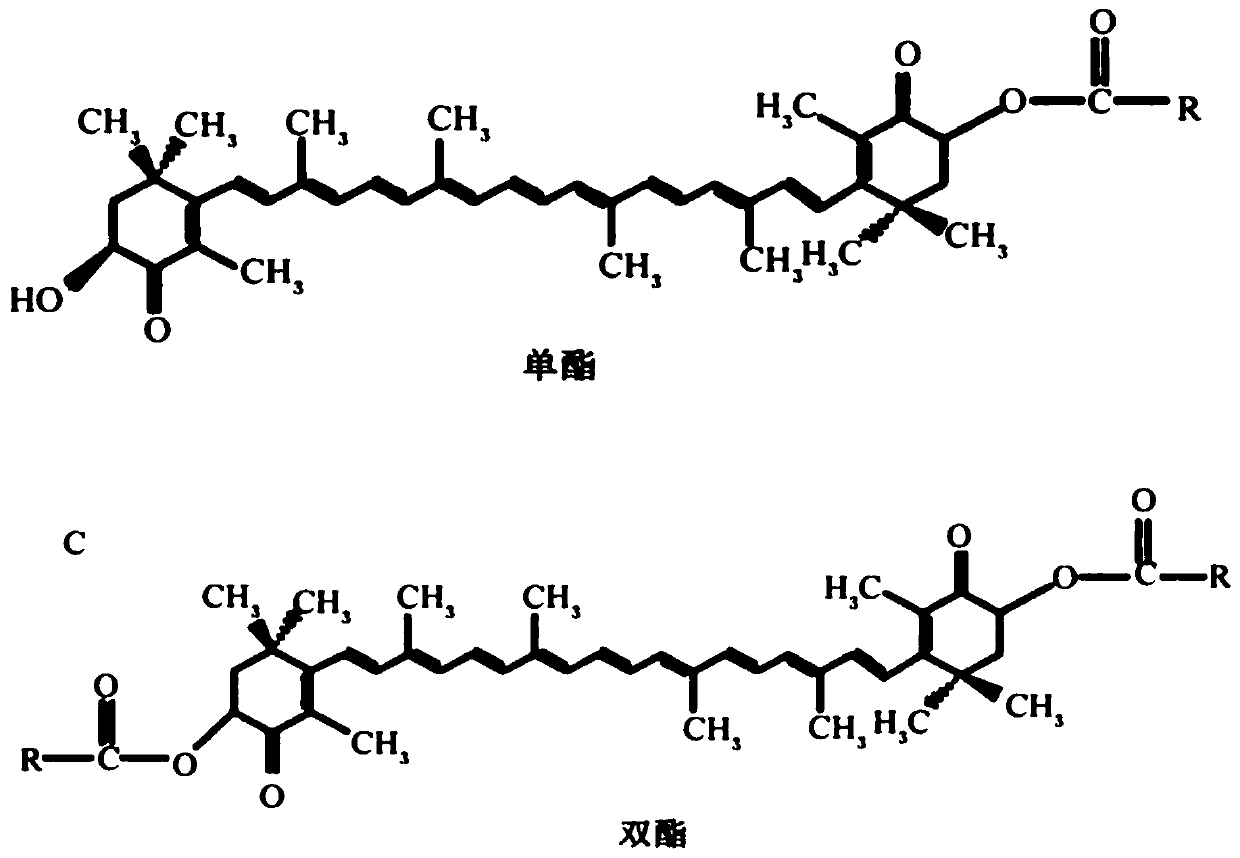
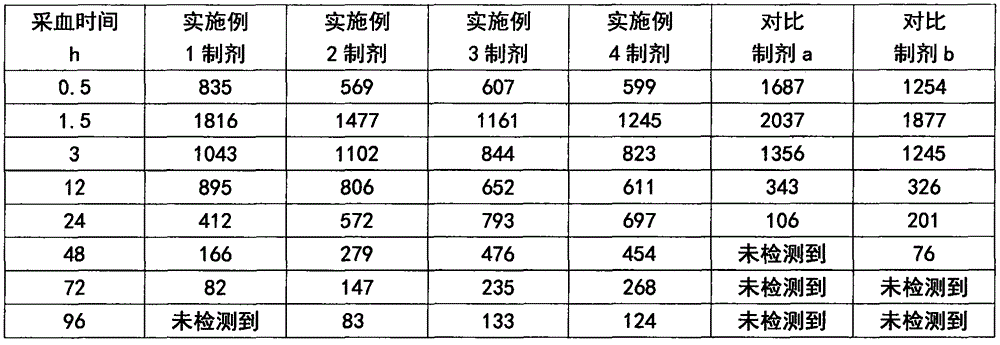
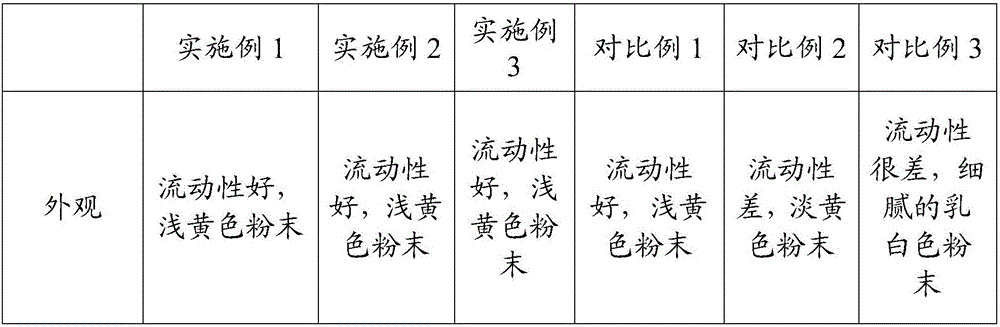
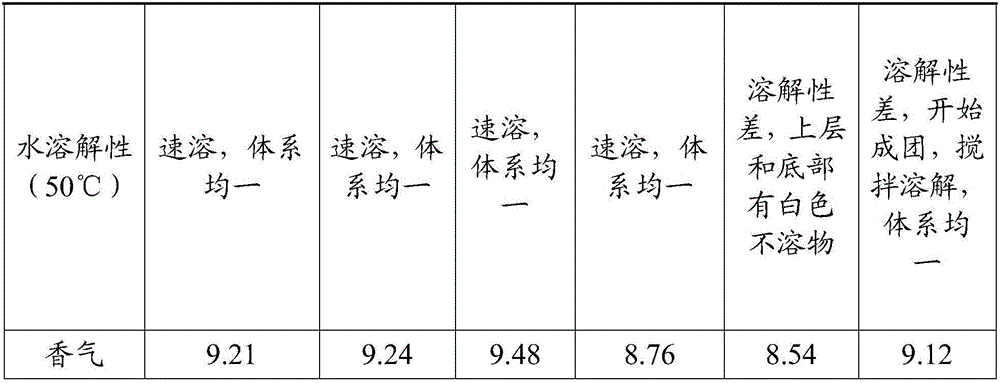
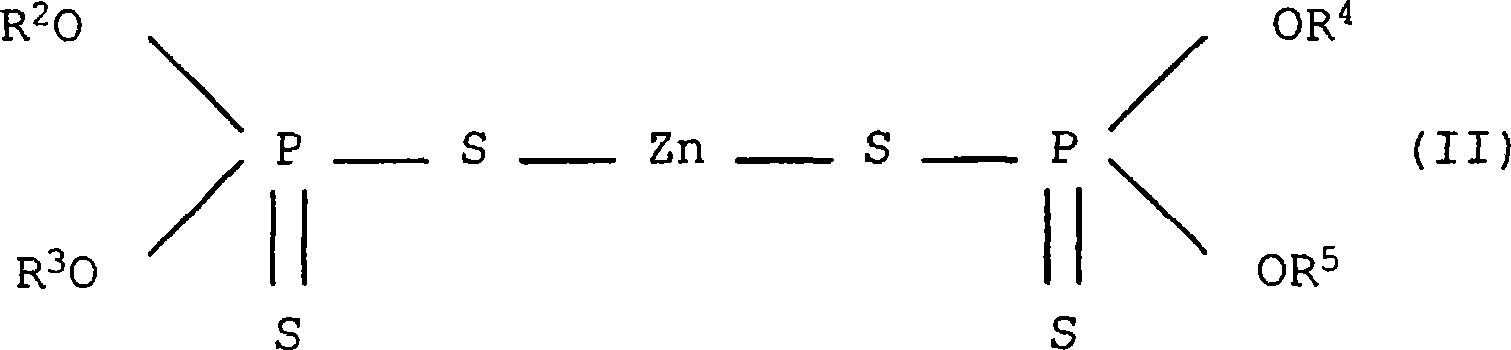Patents
Literature
102 results about "Glycerol monooleate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Glycerol monooleate (C21H40O4) is a clear amber or pale yellow liquid. It is an oil soluble surfactant and is classified as a monoglyceride. It is used as an antifoam in juice processing and as a lipophilic emulsifier for water-in-oil applications.
Thermoplastic food casing
A porous food casing consisting essentially of a film of food grade thermoplastic having a plurality of interconnected interstices therein. The interstices are defined by a porosity modifier selected from the group consisting of soybean oil, peanut oil, corn oil, glycerin, polyethylene glycol, monolaurate, mineral oil, polyoxyethylene, sorbitan monostearate, sorbitan monooleate and glycerol monooleate. The interstices are in a range of approximately 0.002 to 1 micron and the casing has a water vapor permeability in a range of about 1 to 1500 gms / m2 / min.
Owner:YEN WILLIAM W
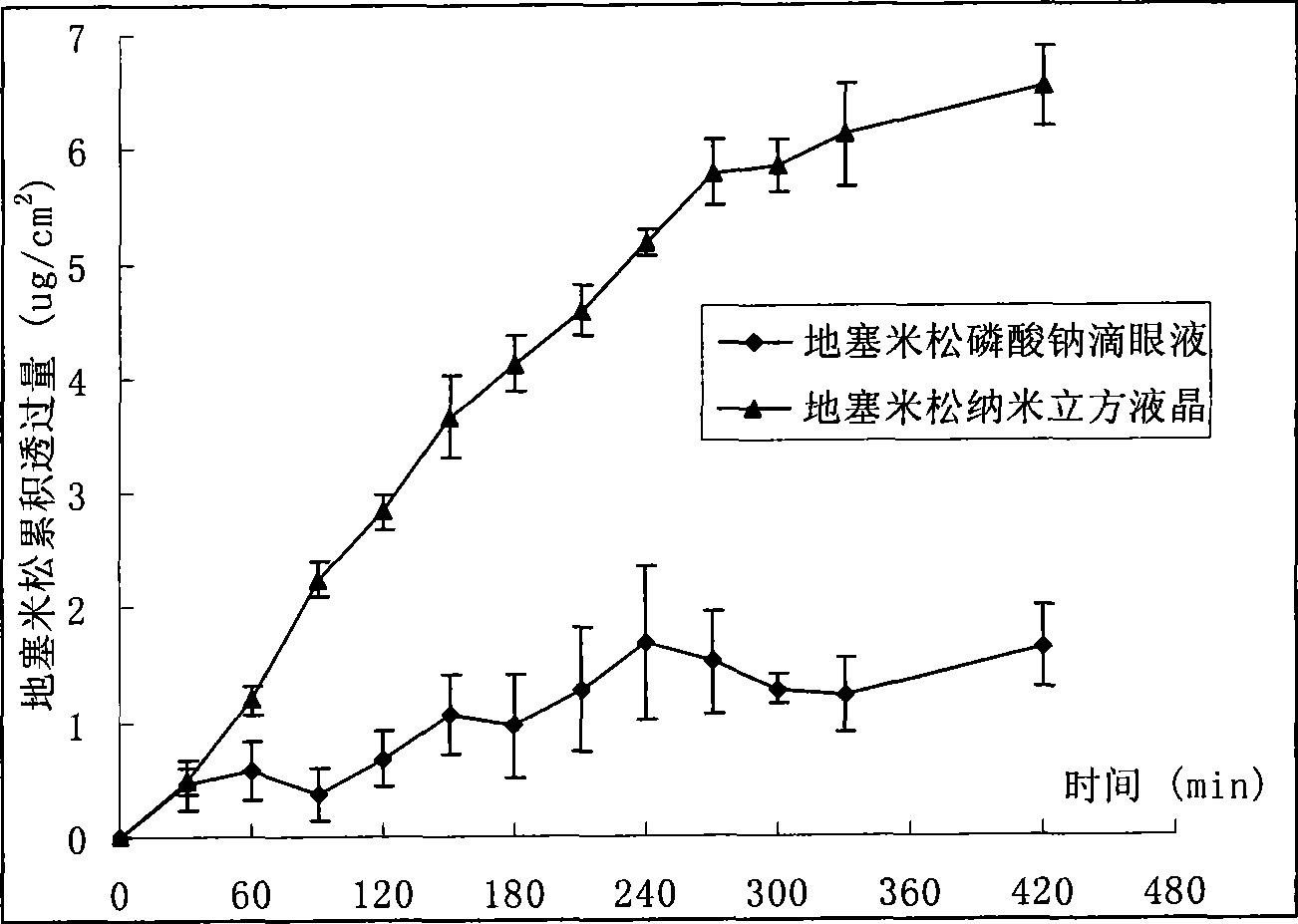
Nano cubic liquid crystal dexamethasone preparation for eye and preparation method thereof
InactiveCN101502485ASolve solubilitySolving Dispersion ProblemsOrganic active ingredientsSenses disorderLipid formationPolyvinyl alcohol
Owner:CHINA PHARM UNIV
Tooth Whitener
InactiveUS20120134936A1Promote disseminationEfficient deliveryCosmetic preparationsToilet preparationsPolyolSolvent
A tooth whitener is provided, including a water-in-oil (W / O) emulsion phase including: a discontinuous phase comprising a peroxide, a hydrophilic solvent, and a polyol, and a continuous phase comprising a glycerol monooleate, a polymer and a polyol, wherein the tooth whitener is flowable upon being applied to teeth and is solidified by the action of moisture after being applied to teeth, and then is adhered and fixed to teeth, and wherein the glycerol monooleate is in an amount of 15% to 95% by weight, based on total weight of the tooth whitener composition.
Owner:LG HOUSEHOLD & HEALTH CARE LTD
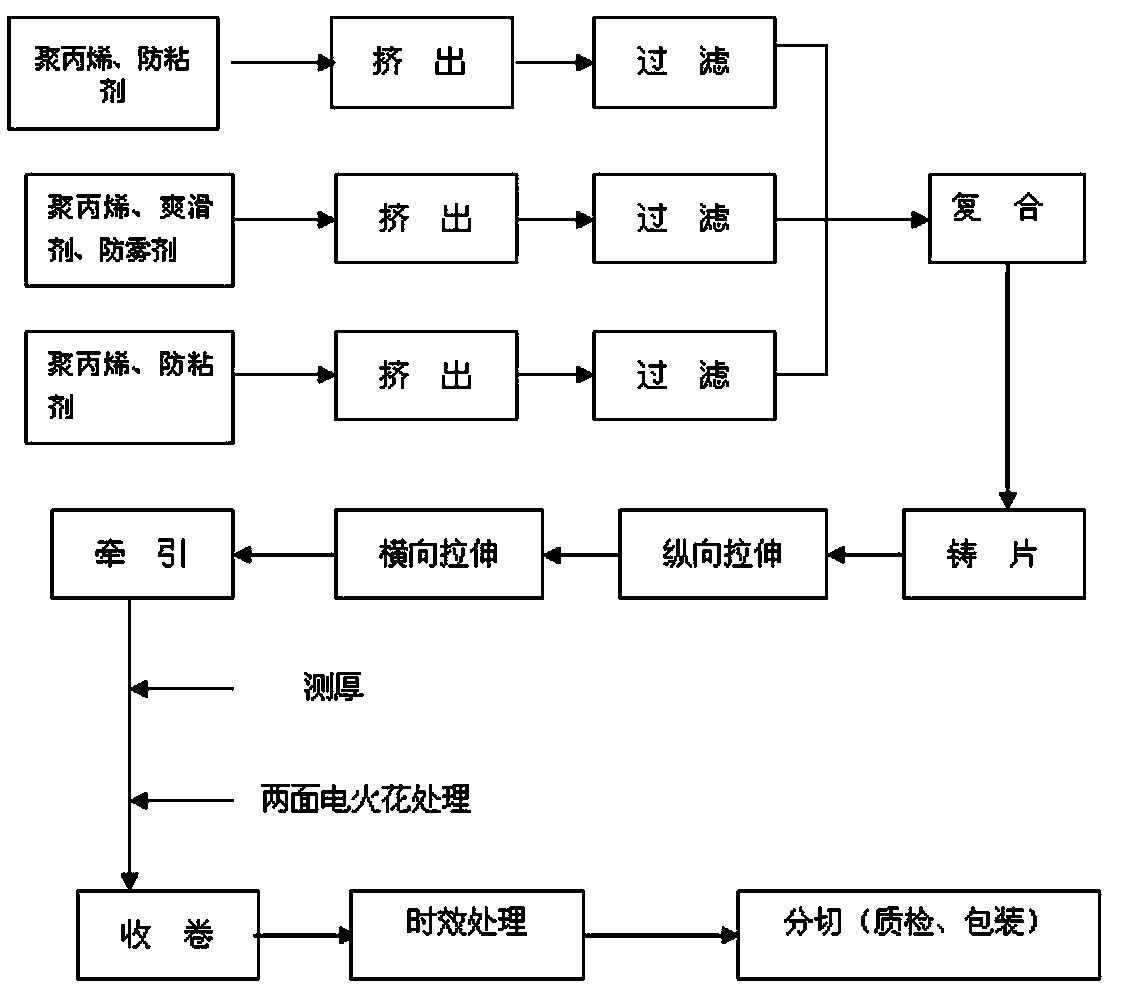
Double-face heat-sealed and anti-fogging type BOPP (biaxially-oriented polypropylene) film and preparation method thereof
ActiveCN103707603AGood anti-fog performanceSlow migration rateSynthetic resin layered productsCoatingsPolymer sciencePolyethylene oxide
The invention relates to a double-face heat-sealed and anti-fogging type BOPP (biaxially-oriented polypropylene) film and a preparation method thereof. The double-face heat-sealed and anti-fogging type BOPP film is formed by co-extruding and biaxially stretching an inner surface layer, a core layer and an outer surface layer, wherein the inner surface layer and the outer surface layer are respectively made from 97.5-98.5 mass percent of polypropylene and 1.5-2.5 mass percent of anti-sticking agent; the core layer consists of 95.9-97.1 mass percent of polypropylene, 2.5-3.5 mass percent of an anti-fogging agent and 0.4-0.6 mass percent of a slipping agent; the anti-fogging agent consists of the following components by weight percent: 50 percent of sorbitan monostearate, 35 percent of glycerin monooleate and 15 percent of polyethylene oxide (20) glycerin monostearate. The selected polypropylene raw material is good in processing performance and high in mechanical strength. The anti-fogging agent is a mixture of multiple fatty acids and is well compatible with BOPP; by the addition of the anti-fogging agent and ethylene oxide, the hydrophilic performance is greatly improved, and the anti-fogging performance is improved.
Owner:ZHEJIANG KINLEAD PACKAGING MATERIAL
Antibacterial micro-pore PE (Poly Ethylene) fresh-keeping film of fruits and vegetables
InactiveCN103304877AImprove adsorption capacityImprove permeabilityLow-density polyethylenePolymer science
The invention relates to an antibacterial micro-pore PE (Poly Ethylene) fresh-keeping film of fruits and vegetables, which belongs to the technical field of daily supplies. The antibacterial micro-pore PE fresh-keeping film of fruits and vegetables is characterized by comprising the following materials in parts by weight: 100-120 parts of P low-density polyethylene resin, 12-18 parts of ethylene-vinyl acetate resin, 9-12 parts of linear polyethylene resin, 0.2-1 part of glyceryl monooleate, 0.5-2 parts of alkyl acid amine ethoxylate, 0.3-0.8 part of antioxidant, 0.05-0.2 part of lavender essential oil, 2-5 parts of titanate coupling agent, 6-10 parts of calcium carbonate and 6-10 parts of kieselguhr. The antibacterial micro-pore PE fresh-keeping film of fruits and vegetables is stable in structure, good in transparency, tensile resistance and softness, good in air permeability and bactericidal power. Meanwhile, the antibacterial micro-pore PE fresh-keeping film of fruits and vegetables has strong adsorption effect and permeability to the gasses including O2, CO2, steam, ethylene and the like.
Owner:SUZHOU NEW DISTRICT JIAHE PLASTIC
Mucoadhesive nanoparticles for cancer treatment
The present invention relates to a pharmaceutical composition which includes nanoparticles. The nanoparticles include a glyceryl monooleate or monolinoleate (or other mono fatty acid ester); a chitosan; and a cancer therapeutic agent, such as gemcitabine, taxanes, and hydrophobic cancer therapeutic agents). Also disclosed are methods for preparing such nanoparticles and pharmaceutical compositions, as well as methods for treating breast, pancreatic, colon, prostate, and other cancers by parenterally, intravenously, or otherwise administering such nanoparticles and pharmaceutical compositions.
Owner:CREIGHTON UNIVERSITY
Anti-wrinkle antibiotic finishing agent as well as preparation method and application thereof
ActiveCN104695219AImprove wrinkle resistanceEnhanced inhibitory effectVegetal fibresBiotechnologyCyclodextrin
The invention discloses an anti-wrinkle antibiotic finishing agent as well as a preparation method and application thereof. The anti-wrinkle antibiotic finishing agent comprises 0.8-2.6wt% of azelaic acid, 1.6-3.2wt% of sodium hydrogen phosphate, 2.5-4wt% of carboxymethyl chitosan, 3.3-5.6wt% of hydroxypropyl-beta-cyclodextrin, 3-6wt% of a perilla frutescens ethyl acetate extract, 3.5-7wt% of a rhizoma kaempferiae ethanol extract, 6-12wt% of polyglycerol monooleate and the balance of water. The preparation method comprises the following steps: (1) preparing the perilla frutescens ethyl acetate extract; (2) preparing the rhizoma kaempferiae ethanol extract; and (3) mixing and stirring the components according to the percentage by weight, and shearing, thereby obtaining the anti-wrinkle antibiotic finishing agent. The anti-wrinkle antibiotic finishing agent has the advantages of good anti-wrinkle effect, good antibiotic effect and wide application range.
Owner:SUZHOU INST OF TRADE & COMMERCE
Oridonin cubic liquid crystal nanoparticle and preparation method thereof
ActiveCN106727336AHigh encapsulation efficiencyOvercome the disadvantage of low oral bioavailabilityPowder deliveryOrganic active ingredientsMass ratioSolvent
The invention relates to an oridonin cubic liquid crystal nanoparticle and a preparation method thereof. The oridonin cubic liquid crystal nanoparticle is prepared from the following raw materials in percentage by weight: 0.1 to 0.5 percent of oridonin, 7 to 64 percent of amphiphilic lipid material, 6 to 29 percent of solvent, 1 to 8 percent of stabilizer, and 25 to 65 percent of water, wherein the amphiphilic lipid material is glycerol mono-oleate or phytould likeriol; the mass ratio of a liquid crystal material to the stabilizer is 1 to (0.01 to 0.30). The oridonin cubic liquid crystal nanoparticle provided by the invention is smaller in particle size, good in uniformity, and beneficial to endocytosis and transfer of enterocyte; by utilizing a unique structure of cubic liquid crystal, a dissolution barrier and a permeation barrier of the oridonin during an oral absorption process are effectively overcome, the oral relative bioavailability of the oridonin is remarkably improved, and meanwhile, the oridonin can be slowly released.
Owner:GUANGZHOU ZHONGDA NANSHA TECH INNOVATION IND PARK +1
Water-in-oil emulsion composition
ActiveCN102361626AImprove emulsion stabilityMakeup adheres wellCosmetic preparationsMake-upAlkaneGlycerol
A water-in-oil emulsion composition which, when used as an emulsion makeup base, can be evenly applied and hence enables cosmetics to be spread well. The emulsion composition has a high internal-aqueous-phase proportion that renders the skin fresh and, despite this, gives a sense of thickness, softness, and a moisturizing effect after application. The emulsion composition further has excellent emulsion stability. The water-in-oil emulsion composition is characterized by comprising the following components (A), (B), and (C) and satisfying the following requirements (1) and (2). Components: (A) glycerol isostearate and / or glycerol oleate (B) an aqueous ingredient (C) an oily ingredient comprising a C20 or lower isoparaffin Requirements: (1) The internal-aqueous-phase proportion obtained by dividing the mass of the aqueous ingredient of component (B) by the total mass of the aqueous ingredient of component (B) and the oily ingredient of component (C) is 68% or higher. (2) The amount of glycerol monoisostearate and / or glycerol monooleate contained in component (A) is 85 mass% or larger with respect to the total amount of (A).
Owner:SHISEIDO CO LTD
Glycerol-containing functional fluid
A functional fluid comprising a major amount of an oil of lubricating viscosity, and greater than about 0.05 wt-% glycerol. A method of preparing a functional fluid comprising adding glycerol to a functional fluid, wherein the glycerol is not glycerol monooleate. A method of preparing an additive concentrate comprising adding glycerol to a diluent oil wherein the concentrate contains from about 1% to about 99% by weight of said diluent. A method of reducing friction comprising contacting a metal surface with a functional fluid comprising a major amount of an oil of lubricating viscosity and greater than about 0.05 wt-% glycerol.
Owner:CHEVRON ORONITE CO LLC
Tooth Whitener
Disclosed herein is a tooth whitener using glycerol monooleate. The tooth whitener is a composition that exhibits teeth whitening effects when being applied to teeth. Specifically, the tooth whitener comprises a glycerol monooleate, a polyol, a polymer, a peroxide and a hydrophilic solvent, and has a W / O emulsion phase. The tooth whitener is flowable before being applied to teeth and is spreadable when being applied to teeth. In addition, the tooth whitener is solidified by the action of moisture, such as saliva, after being applied to teeth, and can thus be adhered and fixed to the teeth. Further-more, changes in viscosity of the tooth whitener with varying temperature can he minimized and the release rate of the whitening ingredient can he controlled by the addition of polyol.
Owner:LG HOUSEHOLD & HEALTH CARE LTD
Glycerol-containing functional fluid
A functional fluid comprising a major amount of an oil of lubricating viscosity, and at least about 0.05 wt-% glycerol. A method of preparing a functional fluid comprising adding glycerol to a functional fluid, wherein the glycerol is not glycerol monooleate. A method of preparing an additive concentrate comprising adding glycerol to a diluent oil wherein the concentrate contains from about 1% to about 99% by weight of said diluent. A method of reducing wear comprising contacting a metal surface with a functional fluid comprising a major amount of an oil of lubricating viscosity and at least about 0.05 wt-% glycerol.
Owner:CHEVRON ORONITE CO LLC
Tooth whitener
ActiveCN101141945ALiquidEasy to operateCosmetic preparationsSolidificationEmulsionAdditive ingredient
Disclosed herein is a tooth whitener using glycerol monooleate. The tooth whitener is a composition that exhibits teeth whitening effects when being applied to teeth. Specifically, the tooth whitener comprises a glycerol monooleate, a polyol, a polymer, a peroxide and a hydrophilic solvent, and has a W / O emulsion phase. The tooth whitener is flowable before being applied to teeth and is spreadable when being applied to teeth. In addition, the tooth whitener is solidified by the action of moisture, such as saliva, after being applied to teeth, and can thus be adhered and fixed to the teeth. Furthermore, changes in viscosity of the tooth whitener with varying temperature can be minimized and the release rate of the whitening ingredient can be controlled by the addition of polyol.
Owner:LG HOUSEHOLD & HEALTH CARE LTD
Diesel engine oil composition for improving fuel efficiency and endurance performance
ActiveUS20160108336A1Improve fuel efficiencyMaximizing friction reductionAdditivesFriction reductionPolystyrene
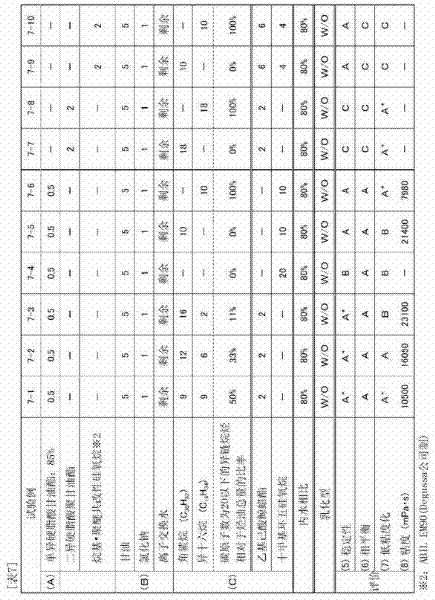
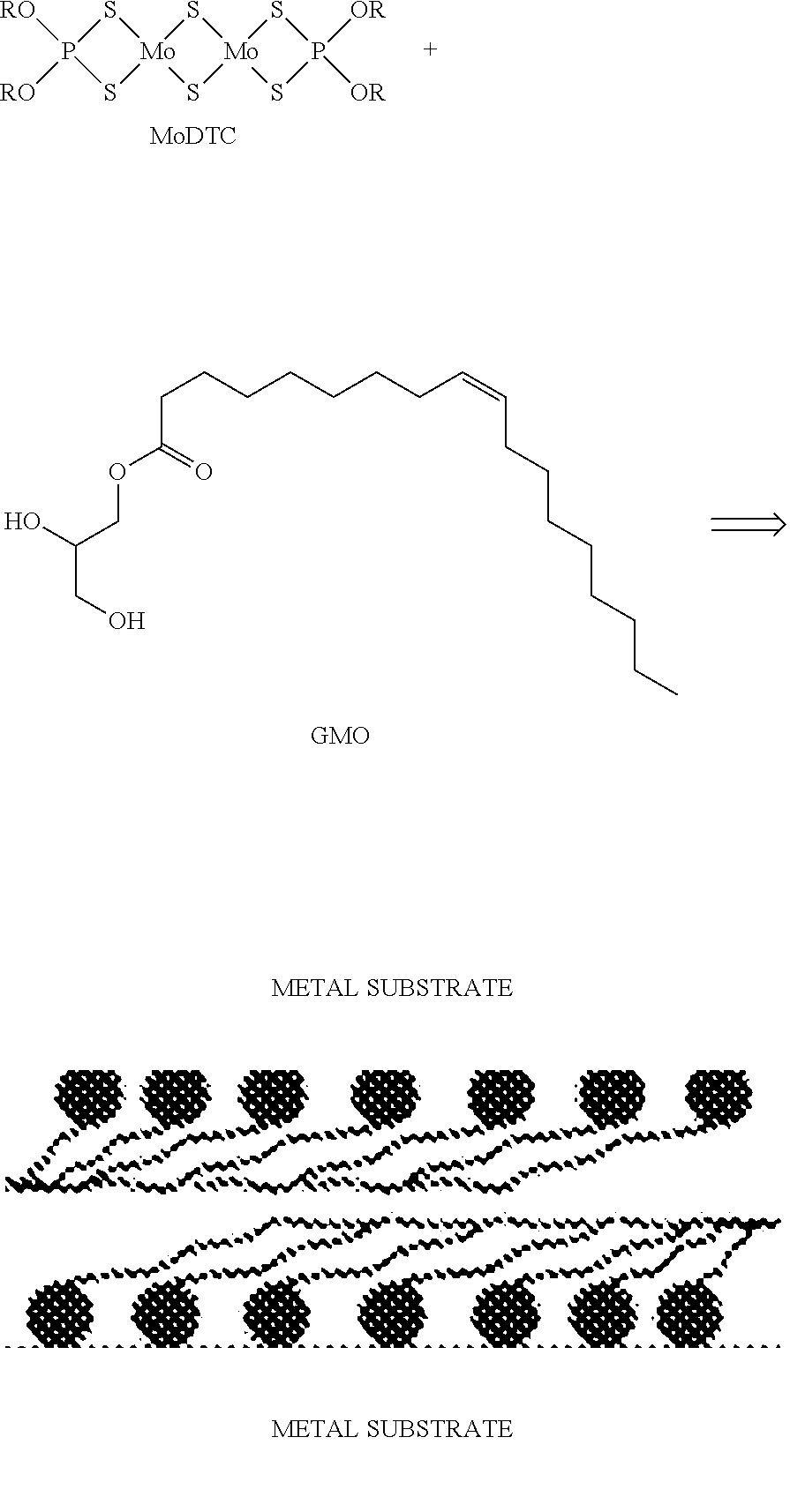
Disclosed is a novel diesel engine oil composition for fuel efficiency and endurance performance. The diesel engine oil composition may maximize frictional resistance and wear resistance by facilitating the formation of a lubricating film on a metal surface, extend an oil change cycle with excellent engine protection ability, and improve fuel efficiency due to friction reduction. The diesel engine oil composition include: base oil; a calcium-based or magnesium-based sulfonate detergent dispersant; a viscosity index improver selected from the group consisting of a diene copolymer, a polystyrene-diene copolymer and a hydrogenated polystyrene-diene copolymer; and a low friction agent including molybdenum dithiocarbamate (MoDTC) and glycerol monooleate (GMO).
Owner:HYUNDAI MOTOR CO LTD
Preparation method and application of sodium stearyl lactate
InactiveCN105152930AThe esterification reaction is completeHigh yieldOrganic compound preparationCarboxylic acid esters preparationSodium lactatePolyethylene glycol
The invention discloses a preparation method of sodium stearyl lactate. The preparation method comprises the following steps: adding lactic acid into water; performing uniform stirring; adding sodium carbonate; performing stirring for 1-1.5h at 55-65 DEG C; adding stearic acid and polyethylene glycol-400; performing a reaction for 2-3h at a temperature of 160-180 DEG C and at a vacuum degree of 0.07-0.09 MPa; discharging the obtained product after the reaction is over; recrystallizing the obtained product with absolute ethanol; and performing vacuum drying to obtain sodium stearyl lactate. The preparation method is high in yield, mild in reaction condition, short in production cycle, low in energy consumption, and less in production cost. At the same time, the invention further provides a stabilizer for a vegetable protein drink, wherein the stabilizer comprises prepared sodium stearyl lactate, propylene glycol monostearate, polyglycerol monooleate, xanthan gum, and Arabic gum. The stabilizer can effectively improve the stability of the vegetable protein drink.
Owner:GUANGZHOU CARDLO BIOCHEM TECH
Veterinary anti-parasitic preparation containing carnauba wax
ActiveCN104666244AThe determination method is simpleOrganic active ingredientsSolution deliveryVegetable oilMethyl oleate
The invention relates to a veterinary anti-parasitic injection containing carnauba wax. The preparation is mainly prepared from anti-parasitic medicine, the carnauba wax and an oily medium, wherein the anti-parasitic medicine is avermectins, praziquantel and oxfendazole; the oily medium is vegetable oil, ethyl oleate or benzyl benzoate, and more than one of the vegetable oil, ethyl oleate or benzyl benzoate can be combined to use. Glycerol mono-oleate can be added in the preparation, the carnauba wax and the glycerol mono-oleate are compositely applied, and the slow-release effect of the preparation is better. The selected preparation is prepared from 36-80 g of abamectin anti-parasitic medicine, 10-70 g of the carnauba wax, 80-120 g of the glycerol mono-oleate, 0.1-0.3 g of an antioxidant, 10-20 g of benzyl alcohol and the balance of the oily medium (per 1000ml).
Owner:中农华威制药股份有限公司
Propolis soft capsule content and preparation method thereof
InactiveCN102512456AGood dispersionEvenly dispersedAnthropod material medical ingredientsMetabolism disorderAlcoholPropolis
The invention discloses a propolis soft capsule content and a preparation method thereof. Propolis soft capsules are prepared by taking glycerol monooleate, glycerol and soyabean lecithin as a propolis carrier through the following steps of: preparing alcohol ester mixed solution; preparing alcohol ester propolis; cooling; preparing hydrophilic propolis liquid; preparing propolis soft capsules and the like. The propolis has high hydrophilicity; auxiliary materials with low safety such as polyethylene glycol and the like are not used; the glycerol monooleate, the glycerol and the soyabean lecithin are used as the propolis carrier; the strict national regulation on use of the health-care food raw materials at present is met; the product is stable; the safety is high; meanwhile, the hydrophilicity of the propolis is enhanced; and the absorption rate of a human body is increased.
Owner:ZHEJIANG JIANGSHAN HENGLIANG BEE PRODS
Glycerol monooleate chitosan liquid crystal polymer
InactiveCN102108102ALiquid crystal compositionsPharmaceutical non-active ingredientsChemical synthesisCrystallography
The invention belongs to the field of medicinal high polymer materials and in particular relates to a chemical synthesis pathway. Glycerol monooleate (GMO) is grafted on a chitosan (2-position amino) to form a glycerol monooleate chitosan liquid crystal polymer. The glycerol monooleate chitosan liquid crystal polymer can serve as a carrier of a liquid crystal medicament delivery system. The GMO chitosan polymer is characterized in that the GMO molecule is grafted on the 2-position amino of a chitosan framework. The GMO chitosan polymer has the structural characteristic that n in the structure is an integer which is more than zero.
Owner:CHINA PHARM UNIV
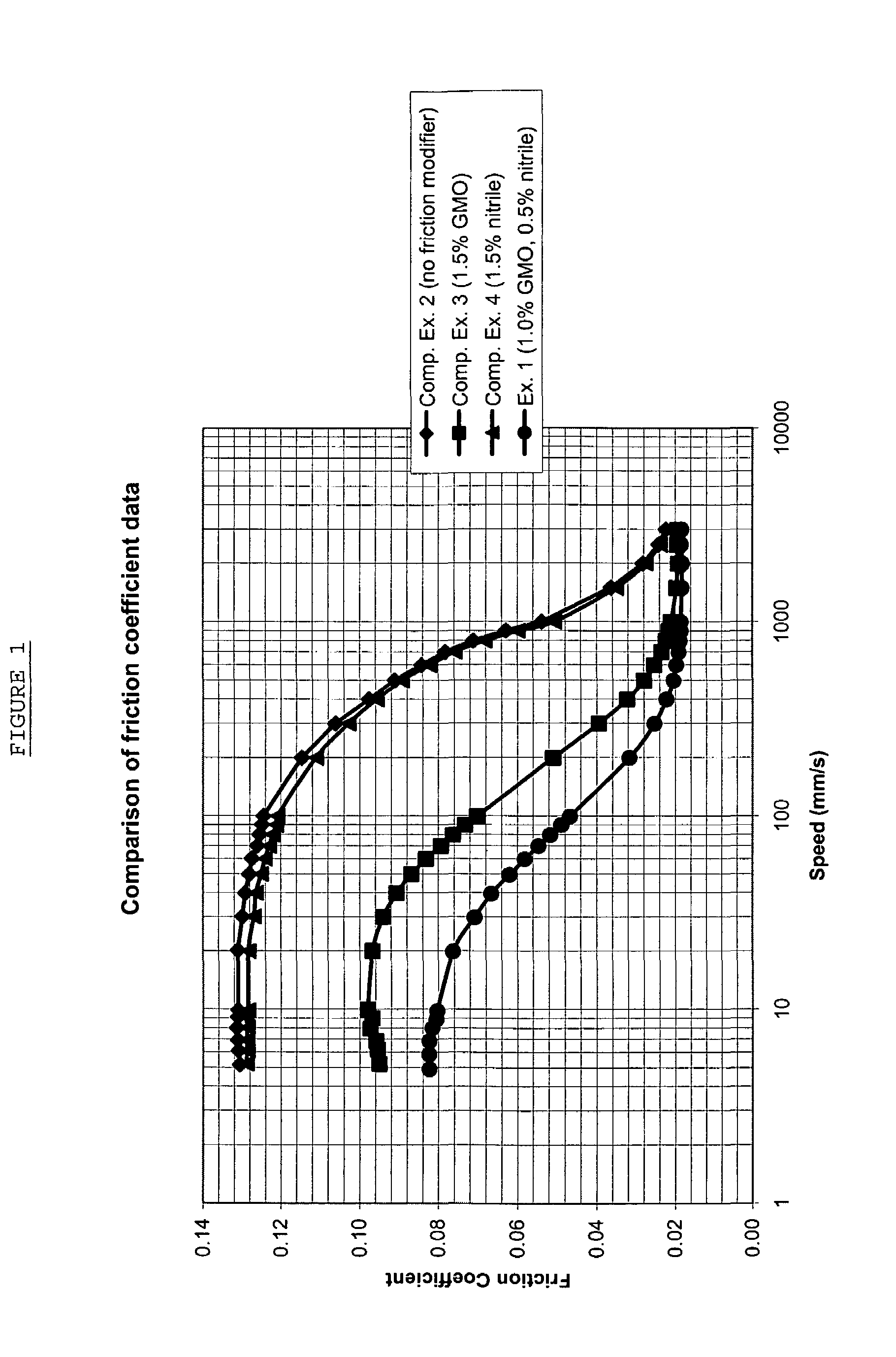
Lubricating oil composition
A lubricating oil composition comprising base oil, glycerol monooleate and one or more nitrile compounds; and a method of lubricating an internal combustion engine comprising applying said lubricating oil composition thereto.
Owner:SHELL INT RES MAATSCHAPPIJ BV
Lubricating oil composition
A lubricating oil composition is disclosed containing base oil, glycerol monooleate and one or more nitrile compounds. A method of lubricating an internal combustion engine is also disclosed.
Owner:SHELL USA INC
Nasal nano-preparation puerarin liquid crystal nanoparticle and preparation method thereof
ActiveCN107184554AImprove permeabilityOrganic active ingredientsPharmaceutical non-active ingredientsHigh cellVitamin E Acetate
The invention provides a nasal drug delivery nano-preparation puerarin liquid crystal nanoparticle and a preparation method thereof. The puerarin liquid crystal nanoparticle comprises the following raw materials: 1-4.5 parts by weight of glycerol monooleate, 1-3 parts by weight of poloxamer 407, 0.2-0.8 part by weight of puerarin, 0.5-2 parts by weight of vitamin E acetate, and the balance 5% glycerin-water. According to the invention, lipid cubic liquid crystal is adopted as the drug carrier to encapsulate puerarin to prepare the puerarin lipid cubic liquid crystal nanoparticle. The puerarin liquid crystal nanoparticle prepared by the method provided by the invention has high encapsulation efficiency, good stability, slower in vitro release than a puerarin aqueous solution, and high cell penetration rate than a puerarin solution, thus proving that liquid crystal nanoparticle is a good drug delivery dosage form for puerarin in treatment of brain diseases.
Owner:BEIJING UNIV OF CHINESE MEDICINE
Efficient detergent for removing oil stains
InactiveCN104099199AProtect the surfaceLittle surface damageSurface-active non-soap compounds and soap mixture detergentsSodium bicarbonateSodium phosphates
The invention discloses an efficient detergent for removing oil stains. The efficient detergent comprises raw components of a formula in parts by weight as follows: 10-12 parts of hydrochloric acid, 5-10 parts of sodium bicarbonate, 8-10 parts of ferric hydroxide, 3-8 parts of aluminum hydroxide, 5-10 parts of sodium peroxide, 5-8 parts of manganese dioxide, 5-10 parts of potassium permanganate, 10-12 parts of sodium chloride, 3-5 parts of sodium phosphate, 10-12 parts of sodium stearate, 5-8 parts of fatty alcohol-polyoxyethylene ether, 5-8 parts of alkyl phenol polyoxyethylene ether, 3-5 parts of sodium hypochlorite, 10-12 parts of isopropanol, 8-10 parts of ethyl acetate, 5-8 parts of sodium carboxymethyl starch, 5-10 parts of sodium polyacrylate, 3-5 parts of hydroxy propyl cellulose, 3-8 parts of sodium aliphatate, 6-10 parts of glyceryl monostearate and 50-75 parts of deionized water. The efficient detergent for removing oil stains can effectively remove stubborn stains such as lampblack and the like so as to protect the surface of an object, and a kitchen utensil and a tile can be kept clean and bright; and the formula is mild, and damage to the surface of the object is small while the stains are removed.
Owner:司徒建辉
Tetrandrine lipid nanoparticle ophthalmic preparation and preparation method thereof
InactiveCN109985024AHuge membrane surface areaHigh drug loadingOrganic active ingredientsSenses disorderSolubilityHigh concentration
The invention discloses a tetrandrine lipid nanoparticle ophthalmic preparation and a preparation method thereof, wherein the preparation method comprises the following steps of: adding glycerol monooleate and tetrandrine into absolute ethyl alcohol, heating, carrying out ultrasonic dissolving and pressure-reducing rotary evaporation, and removing all the ethyl alcohol to obtain an oil phase; (2)placing a slow-release material, a penetration enhancer and a cationic material into ultrapure water, stirring, heating and dissolving to obtain a water phase; (3) adding the water phase into the oilphase, shearing, dispersing and cooling to the room temperature to obtain colostrum; and carrying out ultrasound to obtain the tetrandrine lipid nanoparticle ophthalmic preparation. The preparation has the advantages that the membrane superficial area is large, the drug loading capacity is big; the resistance time of drugs in eyes is prolonged, the bioavailability is improved, the eye irritation and the systemic toxicity caused by repeated administration and high concentration of the drugs are avoided. The cationic material can be degraded in vivo. The long-term stability of the preparation ishigh, the solubility is improved and the drug absorption is enhanced. The preparation has a microstructure similar to a biological membrane and is suitable as a carrier of a mucosal drug delivery system.
Owner:TIANJIN UNIV OF TRADITIONAL CHINESE MEDICINE
Fresh-scent type tobacco sheet tobacco shreds and preparing method thereof
InactiveCN105747265AHigh strengthImprove water resistanceTobacco preparationBarley oilOCTENYLSUCCINIC ACID
The invention discloses fresh-scent type tobacco sheet tobacco shreds.The fresh-scent type tobacco sheet tobacco shreds are prepared from, by weight, 40-60 parts of tobacco residues, 20-30 parts of tobacco stems, 3-5 parts of herba taraxaci, 2-4 parts of honeysuckle flowers, 0.5-1 part of octenyl succinic anhydride, 0.05-0.1 part of xanthan gum, 0.5-1 part of avocado oil, 0.1-0.2 part of barley oil, 3-5 parts of Chinese yam juice, 5-10 parts of calcium carbonate, 2-4 parts of butyl titanate and 0.2-0.4 part of glycerol monooleate.The fresh-scent type tobacco sheet tobacco shreds are high in tensile strength, folding strength and water resistance, not likely to break or go bad, fresh and mellow in taste, and capable of reducing smoke irritation, relieving discomfort of the throat and improving smoking quality and have good processability, quality stability and organoleptic quality, and the use value is increased.
Owner:CHUZHOU CIGARETTE MATERIALS FACTORY
Sunscreen emulsion
ActiveCN103040694ALower pass rateImprove sun protection effectCosmetic preparationsToilet preparationsBenzoic acidPolymer science
The invention belongs to the technical field of cosmetic, and in particular relates to a sunscreen emulsion. The sunscreen emulsion is characterized by comprising the following components: anthranilic acid menthyl acetate, para aminobenzoic acidisobutyl ester, octyl hydroxystearate, four glycidyl mono-fatty acid ester, polypropylene oxide octadecyl ester, aluminium stearate, Vaseline, paraffins, mineral oil, thioester magnesium, methylparaben, propylparaben, cactus liquid, essence, ethyecellulose N-10, and deionized water. According to the sunscreen emulsion, the problem of low sunscreen property in the prior art is solved. The sunscreen emulsion has the beneficial effects that the sunscreen index of the sunscreen emulsion is more than 25, the ultraviolet rays passing rate is less than 0.5%, the sunscreen property is much higher than that of the sun block, a sun-screening agent, and sun cream in the prior art, and the property of the product is stable and the manufacture method is simple.
Owner:苏州工业园区安诺科斯化妆品研发有限公司
Water-soluble haematococcus pluvialis astaxanthin soft capsules and preparation method therefor
PendingCN111567805AGood water solubilityIncrease disintegration rateFood homogenisationFood preservationSaturated fatty acid esterCellulose
The invention discloses water-soluble haematococcus pluvialis astaxanthin soft capsules and a preparation method therefor. Capsule walls of the astaxanthin soft capsules comprise the ingredients in parts by mass: 100 parts of gelatin, 6 parts of citric acid, 20 parts of glycerine, 4 parts of sodium carboxymethyl cellulose, 10 parts of sorbitol and 11-13 parts of water; and capsule cores comprise the ingredients in percentage by mass: 10.0%-18.0% of haematococcus pluvialis astaxanthin oil, 10.0%-20.0% of polyethylene glycol-400, 20.0%-40.0% of mono- / bis-glyceryl unsaturated fatty acid ester, 2.0%-5.0% of sorbitol and 30.0%-45.0% of decapolyglyceryl monooleate. The preparation method for the astaxanthin soft capsules further comprises a step of carrying out high-pressure homogenizing mixingunder nitrogen protection so as to prepare the capsule cores. According to the water-soluble haematococcus pluvialis astaxanthin soft capsules and the preparation method therefor, all adjuvants employed are used by health-care foods according to production needs, an emulsifier is an unsaturated fatty acid processed product, the astaxanthin soft capsules are safe, non-toxic, compliant and legal, are low in health security risk and are suitable for an aqueous nutrient absorption system of the intestines and stomach of a human body, and the bioavailability of an active ingredient, i.e., astaxanthin can be improved.
Owner:YUNNAN AIERKANG BIOTECH
Rare-earth luminescent spun-bonded non-woven fabric and production method and composition thereof
The invention discloses a rare-earth luminescent spun-bonded non-woven fabric and a production method and composition thereof. The rare-earth luminescent spun-bonded non-woven fabric comprises, by weight, 90-110 parts of slices, 3-30 parts of SrAl2O4:Eu2+, Dy3+, 0.01-0.03 part of glycerin mono-fatty acid ester, 0.02-0.04 part of phosphite ester, 0.01-0.05 part of 2-hydroxide radical-4-octyl oxygen radical hydroxyl diphenyl ketone, 0.03-0.04 part of polyving akohol and 0.1-0.5 part of methylcellulose. The production method comprises the steps that firstly, the SrAl2O4:Eu2+, Dy3+, the glycerin mono-fatty acid ester, the phosphite ester, the 2-hydroxide radical-4-octyl oxygen radical hydroxyl diphenyl ketone, the polyving akohol and the methylcellulose are added to the dried slices to be fused, and a fused solution is obtained; secondly, the fused solution is added to a screw extruder to be extruded out in a fusion state, and a plurality of continuous filaments are produced through a spinneret plate and are drafted and collected to a lapping machine; lastly, the rare-earth luminescent spun-bonded non-woven fabric is produced by means of hot-pressing through a hot-press roller. A rare-earth aluminate luminescent material is good in chemical stability and heat stability, low in production cost, free of radioactivity and safe and harmless to the human body.
Owner:扬州江辉纺织科技有限公司
Carnauba wax-containing valnemulin injection
ActiveCN104906590AAntibacterial agentsPharmaceutical delivery mechanismGlyceryl monostearateSodium citrate dihydrate
The invention relates to a carnauba wax-containing valnemulin injection with latex-like appearance. Each liter of the injection comprises 150-180g of valnemulin hydrochloride, 2.5-11g of carnauba wax, and the balance of glyceryl triacetate / benzyl benzoate / 1,2-propylene glycol / ethanol with a volume ratio of 4.5:3:3.3:1. Each of the injection also comprises 100-150ml of glyceryl monooleate. In the preparation process of 1L of the injection, 13.0-31.2g of sodium citrate dihydrate (C6H5Na3O7.2H2O) or 19.0-31.9g of sodium carbonate decahydrate (Na2CO3.10H2O) is added. Added sodium citrate or sodium carbonate reacts with valnemulin hydrochloride. Each liter of the injection prepared in the invention includes 150-180g of valnemulin alkali and valnemulin acid salt (hydrochloride or citrate), 2.5-11g of carnauba wax, 100-150ml of glyceryl monooleate, and the balance of glyceryl triacetate / benzyl benzoate / 1,2-propylene glycol / ethanol with a volume ratio of 4.5:3:3.3:1.
Owner:北京中农华威科技集团有限公司
Cheese powder flavor and preparation method thereof
ActiveCN106262654AImprove liquidityReduce moisture contentFood ingredient as flavour affecting agentFood shapingSodium CaseinateOCTENYLSUCCINIC ACID
The invention relates to cheese powder flavor and a preparation method thereof. The cheese powder flavor is prepared from the following raw materials in parts by weight: 3-5 parts by weight of a sulfur-containing flavor ester composition, 0.03-0.05 part by weight of glycerol monooleate, 50-60 parts by weight of a casein hydrolysate, 3-5 parts by weight of starch sodium octenylsuccinate, 2-3 parts by weight of sodium caseinate, 75-80 parts by weight of maltodextrin and 12-20 parts by weight of water. The cheese powder flavor is good in fluidity and low in water content, so that an anti-caking agent does not need to be added into the cheese powder flavor; the cheese powder flavor is instantly soluble, and can be widely applied to leisure food, dairy products, bakery products and the like for providing soft and natural cheese flavor.
Owner:GUANGZHOU FLOWER FLAVOURS & FRAGRANCES
Antibacterial waterproof polyvinyl alcohol nanofiber membrane
InactiveCN106012292AImprove water resistanceImprove stabilityMonocomponent synthetic polymer artificial filamentConjugated synthetic polymer artificial filamentsPhosphomolybdic acidPolyetherimide
The invention discloses an antibacterial and water-resistant polyvinyl alcohol nanofiber film, the raw materials of which include: polyvinyl alcohol, polystyrene, polyetherimide, polyethylene glycol, methanol, glucomannan, cinnamaldehyde, seawater Piolite, rectorite, modified montmorillonite, polydimethylsiloxane, tannin, phosphomolybdic acid, glycerol monooleate, triethyl citrate, polyacrylic acid, phthalic acid, amino acid, N-vinyl-2-pyrrolidone, poly(L-r-chloroethylglutamate), soy protein, water. The antibacterial and water-resistant polyvinyl alcohol nanofiber membrane proposed by the present invention has excellent water resistance, and has excellent antibacterial and anti-pollution properties.
Owner:安庆市天虹新型材料科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com