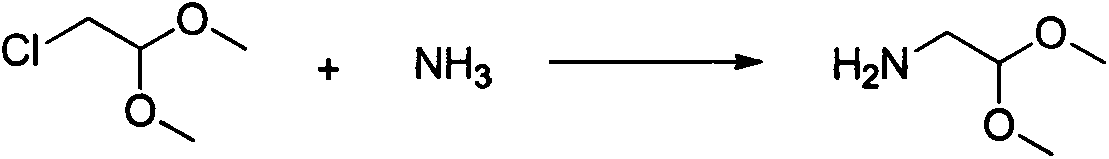Method for preparing praziquantel
A technology of praziquantel and intermediates, applied in the direction of organic chemistry, etc., can solve the problems of unsuitable industrial production, high production cost and high price, and achieve the effects of low cost, good operability and safe feeding method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Add 124.6g (1mol) of chloroacetaldehyde dimethyl acetal and 1280g of 20% ammonia solution to the autoclave, raise the temperature of the reaction to 135-140°C, stir the reaction for 3 hours, distill the reaction solution, and recover the ammonia water until no obvious fraction distills. Until it comes out, weigh 110g of 40% liquid caustic soda aqueous solution and add it to the remaining reaction solution, adjust the pH of the reaction solution to 12-14, distill under reduced pressure, collect 70g of colorless and transparent liquid, and obtain the key raw material aminoacetaldehyde dimethyl acetal, which is used for Intermediate 2 was prepared.
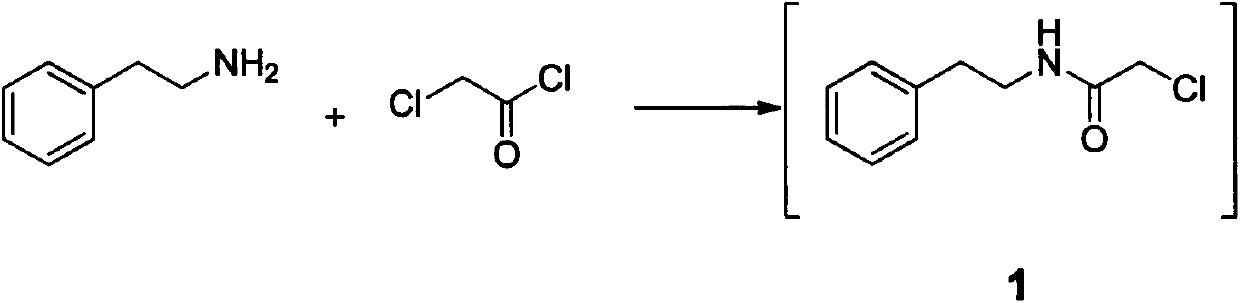
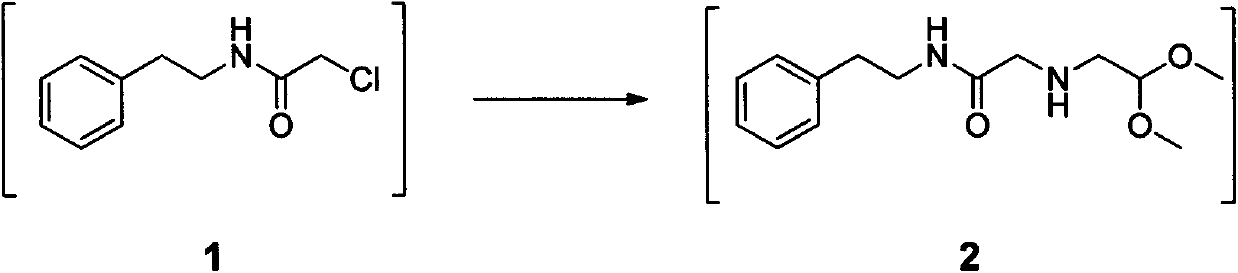
[0032] In the reaction flask, add 24.2g (0.2mol) β-phenylethylamine, 100ml methylene chloride and 60g (0.11mol) 20% Na 2 CO 3 Aqueous solution, stir the mixture until uniform, slowly add 22.6g (0.20mol) of chloroacetyl chloride dropwise under temperature control at -5-0°C, after the addition is complete, continue to control t...
Embodiment 2
[0037] Add 124.6g (1mol) of chloroacetaldehyde dimethyl acetal and 1150g of 30% ammonia solution (20mol) in the autoclave, the reaction is heated up to 135-140°C, stirred for 3 hours, the reaction solution is distilled, and ammonia water is recovered until no Until the obvious fraction evaporates, weigh 110g of 40% liquid caustic soda aqueous solution and add it to the remaining reaction solution, adjust the pH of the reaction solution to 12-14, distill at normal pressure, collect 90g of colorless transparent liquid, and obtain the key raw material aminoacetaldehyde dimethyl acetal , used directly to prepare intermediate 2
[0038] Add 24.2gβ-phenylethylamine (0.2mol), 100ml toluene and 220g10% K in the reaction flask 2 CO 3 (0.16mol) aqueous solution, stir the mixture until uniform, slowly add 56.5g (0.50mol) chloroacetyl chloride dropwise under temperature control at -5-0°C, after the addition is complete, continue to control the temperature and stir for 3 hours, then separ...
Embodiment 3
[0043] Add 124.6g (1mol) chloroacetaldehyde dimethyl acetal and 800g40% ammonia solution (18.8mol) in the autoclave, the reaction is warmed up to 135-140 ° C, stirred for 3 hours, the reaction solution is distilled, and the ammonia is recovered until Until no obvious fractions are distilled out, weigh 110g of 40% liquid caustic soda solution and add it to the remaining reaction solution, adjust the pH of the reaction solution to 12-14, distill the solution under normal pressure, collect 92g of colorless transparent liquid, and obtain the key raw material aminoacetaldehyde Dimethyl acetal, ready for the preparation of intermediate 2.
[0044] Add 24.2gβ-phenylethylamine (0.2mol), 100ml methylene chloride and 83g20% K in the reaction flask 2 CO 3 (0.12mol) aqueous solution, stir the mixture until uniform, slowly add 18.0g (0.16mol) chloroacetyl chloride dropwise at -5-0°C under control of the temperature, after the addition is complete, continue to control the temperature and sti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com