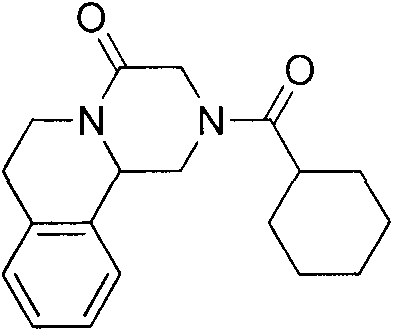
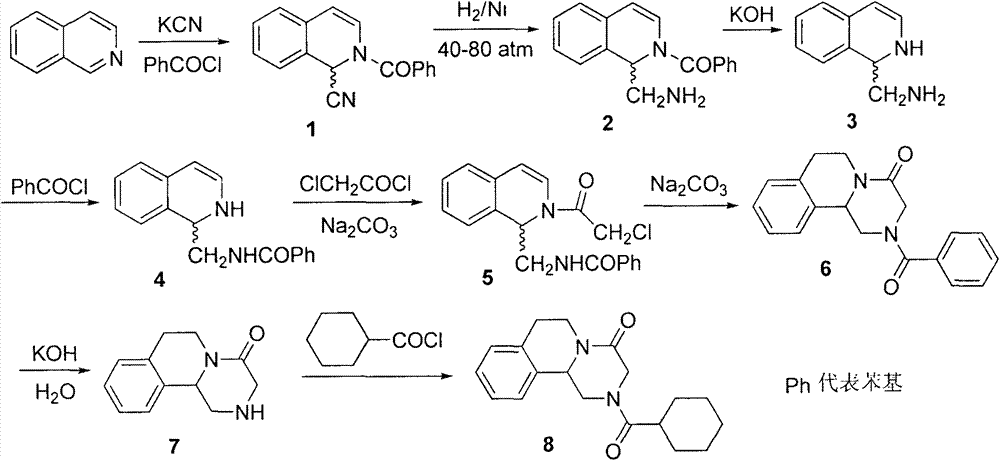
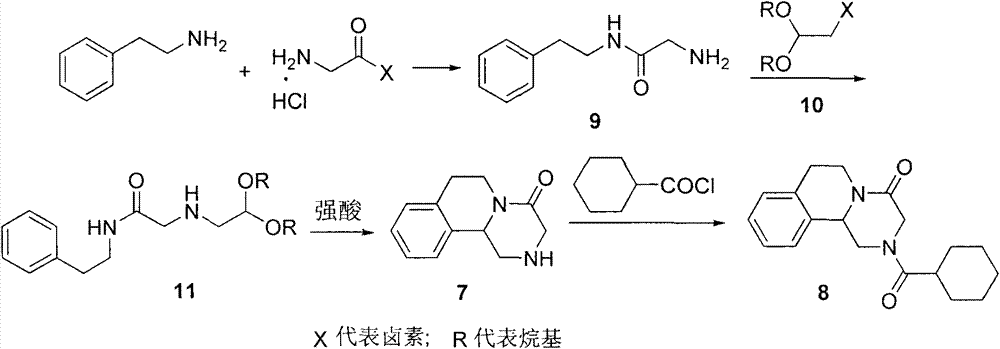
Preparation method of praziquantel
A technology of praziquantel and compound, which is applied in the field of preparation of veterinary drug praziquantel, can solve the problems of high price and not very easy to obtain, and achieve the effects of reducing environmental pressure, simple operation and cost saving
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1: the preparation of compound 12
[0037] Add 122g of phenylethylamine (1mol) and 92.4g of sodium bicarbonate (1.1mol) into 600mL of dichloromethane and stir, cool down to about 0°C in an ice bath, slowly add 124.3g of chloroacetyl chloride (1.1mol) and 120g of dichloromethane For the mixed solution of methyl chloride, after the dropwise addition, heat up to 25°C to continue the reaction for 3h, add 200mL of water to the reaction solution at 0-5°C, separate the organic layer, extract the aqueous layer with dichloromethane (100mL*2), and combine After the dichloromethane layer, dry it with anhydrous sodium sulfate, filter, and distill off the dichloromethane under reduced pressure to obtain 189 g of a white solid with a yield of 96% and a melting point of 120-125°C.
Embodiment 2
[0038] Embodiment 2: the preparation of compound 13
[0039] Add 19.7g (0.1mol) of compound 12 and 12.1g of triethylamine (0.12mol) to 100mL of methanol, stir for 10min, add 9g (0.12mol) of glycine, heat up and reflux and stir for 8h, evaporate the methanol under reduced pressure, and add to the residue Add 50mL of water and 100mL of dichloromethane and stir, let stand to separate layers, separate the dichloromethane layer, wash the water layer with dichloromethane (50mL*2), combine the dichloromethane phases, dry over anhydrous sodium sulfate, and filter with suction. The methylene chloride was distilled off to obtain a brown solid, which was recrystallized from acetone to obtain 20.1 g of an off-white solid with a yield of 85% and a melting point of 187-189°C.
Embodiment 3
[0040] Embodiment 3: the preparation of compound 14
[0041] ①Add 23.6g of compound 13 (0.1mol) and 20.7g of potassium carbonate (0.15mol) to 100g of water and 300mL of dichloromethane, cool to 0°C under stirring, add dropwise 17.5g (0.12mol) of cyclohexanecarbonyl chloride, After the dropwise addition was completed, the temperature was raised to room temperature and stirred for 3 h, the dichloromethane layer was separated, the water layer was extracted with dichloromethane (100 mL*2), the dichloromethane layers were combined, dried over anhydrous sodium sulfate, suction filtered, and the dichloromethane was evaporated under reduced pressure. Chloromethane, add 100mL of petroleum ether to the residue, stir at room temperature for 1h, and suction filter to obtain 30.5g of off-white solid, yield 88%, melting point: 166-168°C.
[0042] ②Add 23.6g of compound 13 (0.1mol) and 6g of sodium hydroxide (0.15mol) to a mixture of 250mL of dichloromethane and 100mL of water, cool to 0°C w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com