Anti-parasitic orally disintegrating tablet for dogs and cats and preparation method thereof
An anti-parasitic and disintegrating tablet technology, which is applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, anti-infectives, etc., can solve the problem of dogs and cats who have difficulty swallowing and do not want to take medicine, and dogs and cats who spit out , Containing for a long time and other problems, to achieve the effect of improving bioavailability, speeding up absorption and reducing pain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
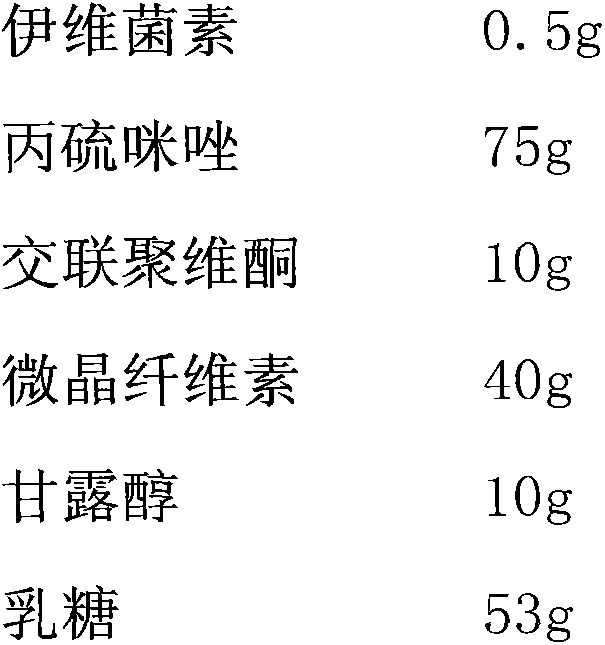
[0019] An anti-parasitic orally disintegrating tablet for dogs and cats, the prescription of the orally disintegrating tablet is based on 1000 tablets of medicine, each tablet weighs 200 mg, a total of 200 g, wherein:
[0020]
[0021]
[0022] An anti-parasitic orally disintegrating tablet for dogs and cats, its preparation method comprises the following steps: (1) raw materials ivermectin, albimazole, crospovidone, microcrystalline cellulose, mannitol, lactose and meat-flavored essence, pass through a 100-mesh sieve respectively for later use, weigh the corresponding quantity according to the formula quantity, and mix them uniformly sequentially in an equal increasing manner.
[0023] (2) Dissolve sodium lauryl sulfate and acesulfame potassium in water, then spray the prepared aqueous solution into the above drug powder, stir quickly, mix evenly to make a soft material, granulate through a 18-mesh sieve, and dry at 60°C , passed through a 20-mesh sieve for granulation;...
Embodiment 2
[0026] An anti-parasitic orally disintegrating tablet for dogs and cats, the prescription of the orally disintegrating tablet is based on 1000 tablets of medicine, each tablet weighs 200 mg, a total of 200 g, wherein:
[0027]
[0028]
[0029] Preparation:
[0030] (1) Pass the raw materials ivermectin, albendazole, crospovidone, microcrystalline cellulose, mannitol, lactose and meat flavor respectively through a 100-mesh sieve for later use, and weigh the corresponding quantity according to the formula , using the method of equal increments to mix uniformly in turn.
[0031] (2) Dissolve sodium lauryl sulfate and acesulfame potassium in water, then spray the prepared aqueous solution into the above drug powder, stir quickly, mix evenly to make a soft material, granulate through a 18-mesh sieve, and dry at 60°C , passed through a 20-mesh sieve for granulation;
[0032] (3) Add micropowder silica gel and magnesium stearate to the above granules, mix well and then compr...
Embodiment 3
[0034] An anti-parasitic orally disintegrating tablet for dogs and cats, the prescription of the orally disintegrating tablet is based on 1000 tablets of medicine, each tablet weighs 200 mg, a total of 200 g, wherein:
[0035]
[0036]
[0037] Preparation:
[0038] (1) Pass the raw materials ivermectin, albendazole, crospovidone, microcrystalline cellulose, mannitol, lactose and meat flavor respectively through a 100-mesh sieve for later use, and weigh the corresponding quantity according to the formula , using the method of equal increments to mix uniformly in turn.
[0039] (2) Dissolve sodium lauryl sulfate and acesulfame potassium in water, then spray the prepared aqueous solution into the above drug powder, stir quickly, mix evenly to make a soft material, granulate through a 18-mesh sieve, and dry at 60°C , passed through a 20-mesh sieve for granulation;
[0040] (3) Add micropowder silica gel and magnesium stearate to the above granules, mix well and then compr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com