Anti-parasitical medicine in situ setting slow release injection and preparation method thereof
A slow-release injection and anti-parasitic technology, which is applied in directions such as pharmaceutical formulations, anti-infectives, and drug combinations, can solve the problems of low drug loading, complicated preparation processes, and low safety of materials used, and achieves a simple preparation method, The effect of good stability and simplified prescription
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
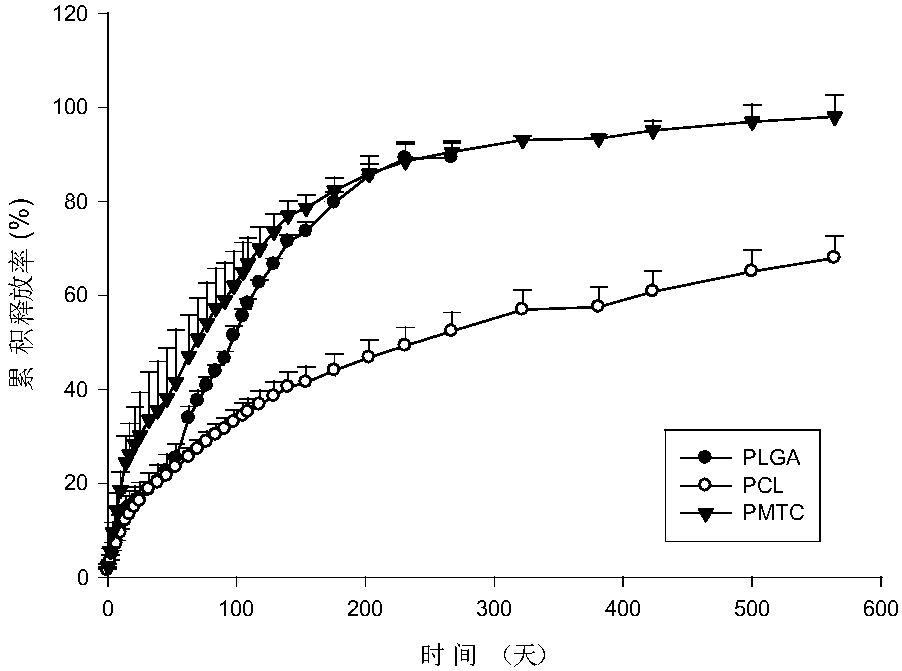
[0063] Different types of polymers were selected to prepare in situ solidified sustained-release injections of antiparasitic drugs with different release periods.
[0064] Drug selection of praziquantel and L-praziquantel, niclosamide, benzimidazoles (including albendazole, mebendazole, flubendazole, fenbendazole and oxfendazole) and ivermectin For the prime, select PLGA, PCL, PTMC as the release skeleton, and its weight-average molecular weight is 16000, and measure the release rate of the drug.
[0065] The preparation method of in-situ solidified sustained-release injection of antiparasitic drugs is as follows: Weigh 0.4 g of the drug, add 0.4 g each of PLGA, PCL and PTMC, and 0.8 mL of NMP, and vortex until the polymer material is completely dissolved to obtain a solution or a mixture. Suspension.
[0066] Take 0.2 mL of in-situ solidified slow-release injection of anti-parasitic drugs, add 15 mL of deionized water, place in a shaker at 37°C, take samples regularly, and tak...
Embodiment 2
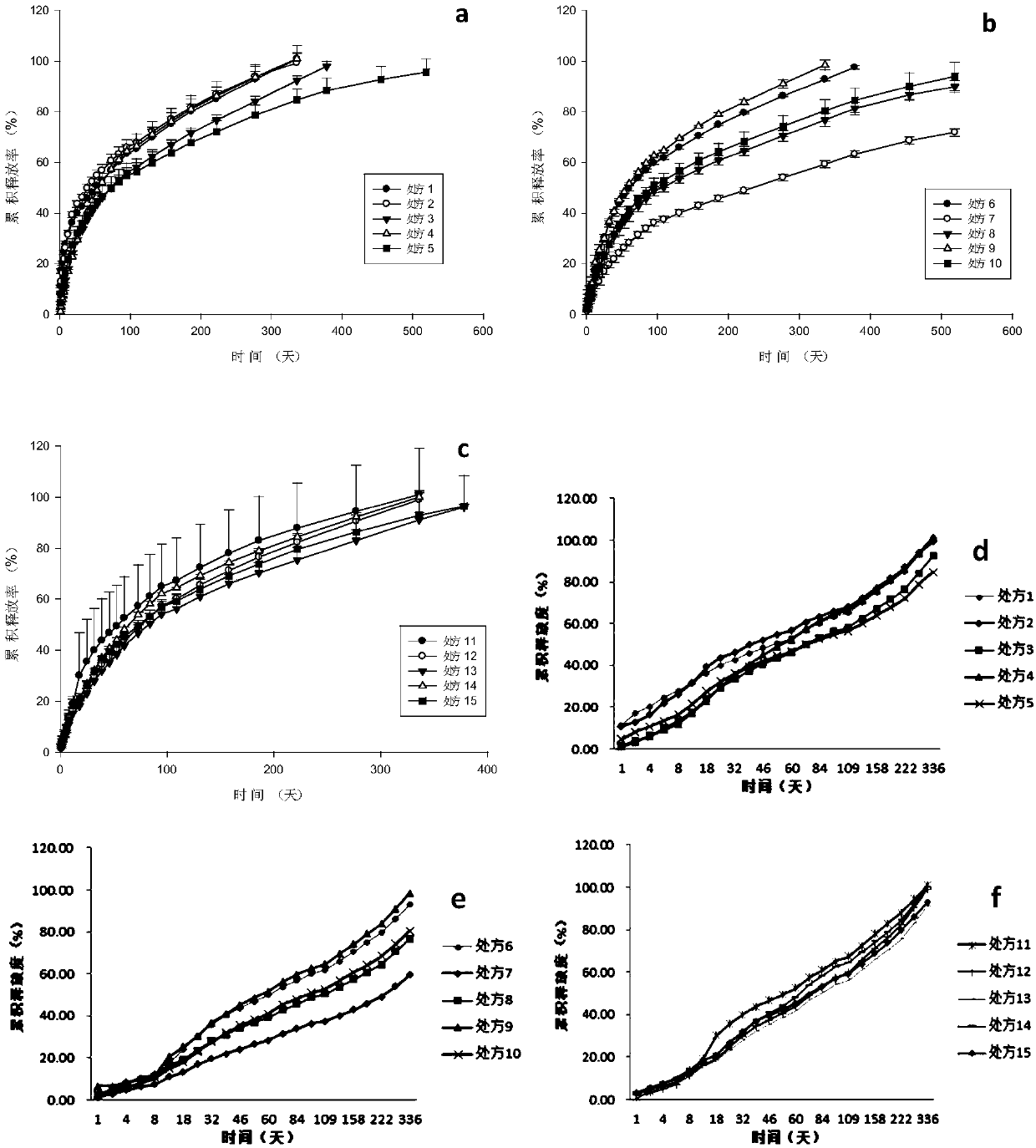
[0069] In situ solidified sustained-release injections of anti-parasitic drugs were prepared with different formulation ratios, wherein the polymer used was PCL, the dispersant was NMP, and the preparation method was the same as in Example 1.
[0070] Taking polymer molecular weight (A), polymer-to-drug ratio (B), and solvent concentration (C) as investigation factors, gel viscosity, water solidification time, and drug release time in vitro as response indicators, the factors and level parameters are shown in Table 1. 15 prescriptions were obtained by Box-Behnken design in the Design Expert 8.0 software, prepared different prescriptions of in-situ solidified sustained-release injections of antiparasitic drugs (3 parallels), and carried out in vitro release tests, the results are shown in figure 2 a- figure 2 f (in the figure, prescription 1-15 corresponds to sample number 1-15), wherein, figure 2 a-2c data analysis with Sigma Plot, figure 2 e-2f uses Excel for data analy...
Embodiment 3
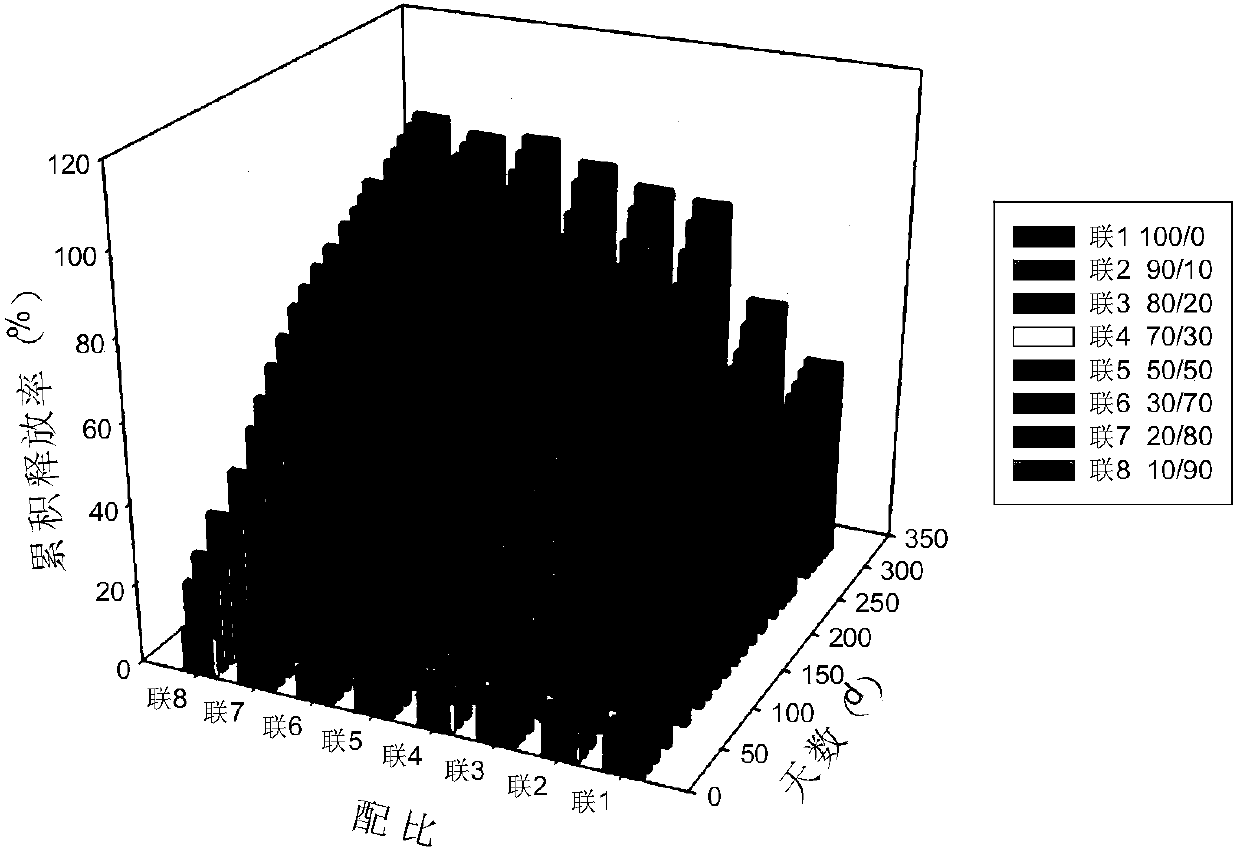
[0078] The release time was adjusted by combining PLGA with different molecular weights.
[0079] Two kinds of PLGA, PLGA with a molecular weight of 150,000 (LA / GA mass ratio: 90 / 10) and PLGA with a molecular weight of 16,000 (LA / GA mass ratio: 75 / 25), were used as the skeleton material in different proportions. The ratio between the degradation rate of the polymer material to control the drug release time.
[0080] Select PLGA (90 / 10) with a molecular weight of 150,000 and PLGA (75 / 25) with a molecular weight of 16,000, respectively according to the weight ratio of 100 / 0, 90 / 10, 80 / 20, 70 / 30, 50 / 50, 30 / 70, 20 / 80 and 10 / 90 are used in combination, see Table 2.
[0081] Table 2 The combination ratio of two PLGAs
[0082] Sample serial number
PLGA (90 / 10, Mw=150,000)
PLGA (75 / 25, Mw=16,000)
Link 1
100
0
couplet 2
90
10
Link 3
80
20
Link 4
70
30
Link 5
50
50
Link 6
30
70
Link ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com