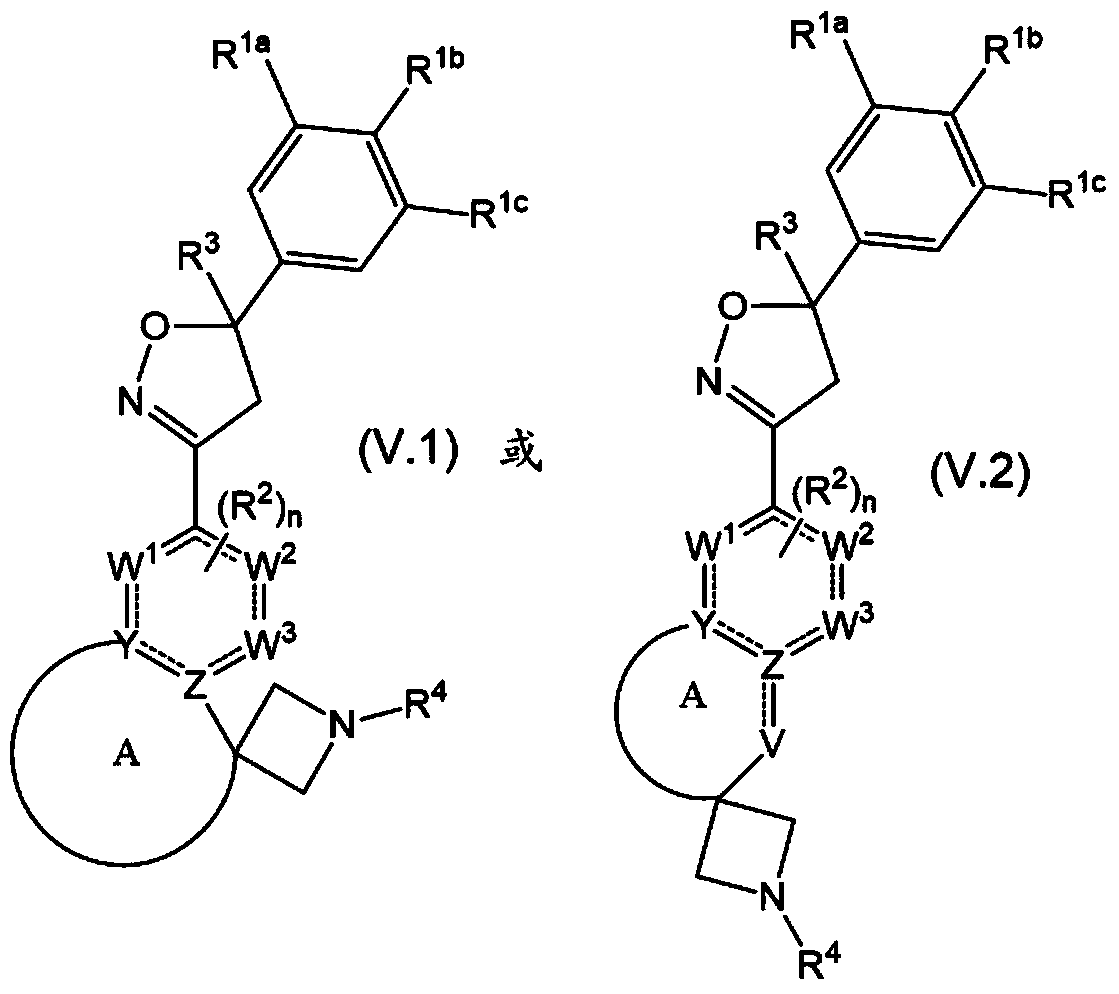
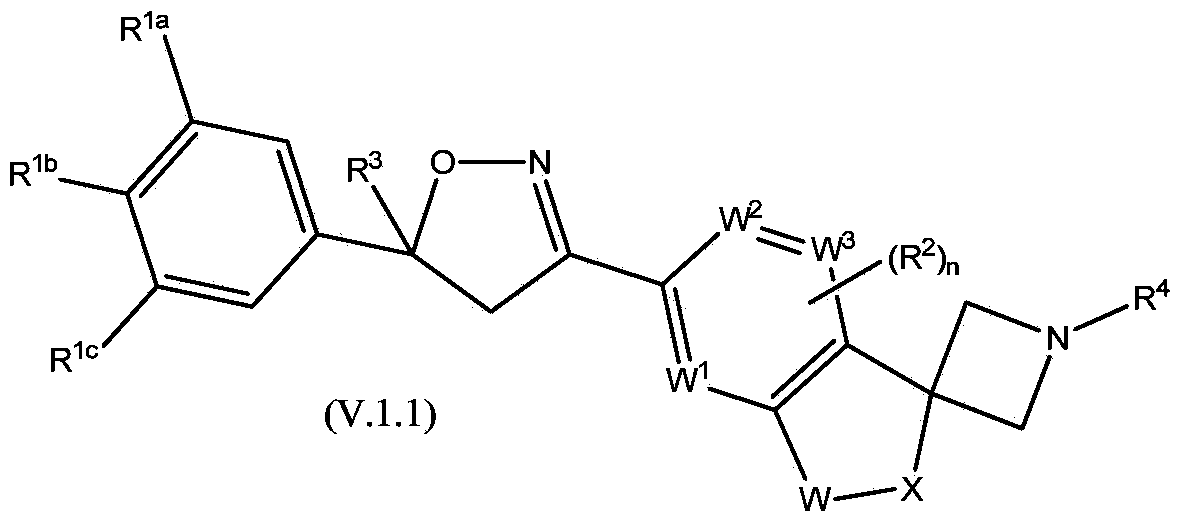
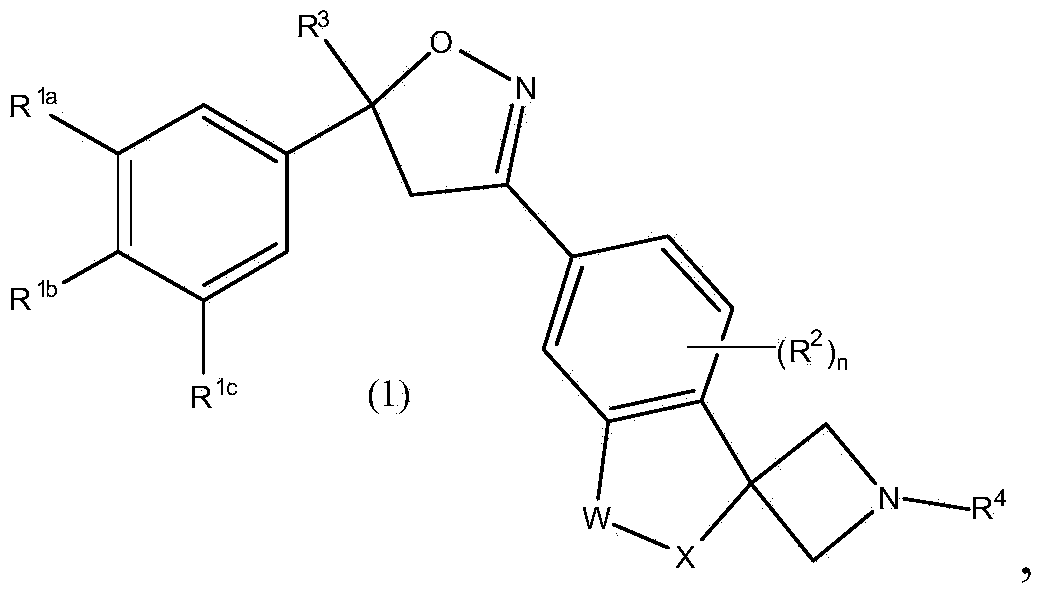
Spirocyclic isoxazoline derivatives as antiparasitic agents
A kind of technology of compound and composition, applied in the field of preparing described spirocyclic isoxazoline derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0537] Example 1: 1-(cyclopropanecarbonyl)-5'-(3,4,5-trichlorophenyl)-5-(trifluoromethyl)-4,5-dihydroisoxazol-3-yl )-3'H-spiro{azetidine-3,1'-isobenzofuran}-3'-one
[0538]
[0539] 5'-(3,4,5-trichlorophenyl)-5-(trifluoromethyl)-4,5-dihydroisoxazol-3-yl)-3'H-spiro{ Azetidine-3,1'-isobenzofuran}-3'-one (140 mg, 0.3 mmol) was dissolved in CH 2 Cl 2 (5 mL), and TEA (0.15 mL, 1.1 mmol) was added. The reaction was stirred at room temperature for 5 minutes, and cyclopropanecarbonyl chloride (35 mg, 0.3 mmol) was added. The resulting solution was stirred at room temperature for 15 minutes. Next, a few drops of MeOH were added, and the reaction was concentrated in vacuo to ~3 mL and injected directly onto a 24 g Redi Sep cartridge. The crude material was chromatographed eluting with 100% heptane to 60:40 EtOAc:heptane to afford the final product (68 mg, 46%) as a solid. 1 HNMR (CDCl 3 )δppm: 8.29(1H), 8.04(1H), 7.85(1H), 7.67(2H), 4.94-4.83(1H), 4.86-4.58(2H), 4.49-4.37(1H), ...
Embodiment 2
[0540] Example 2: 5'-(5-(3,5-dichloro-4-fluorophenyl)-5-(trifluoromethyl)-4,5-dihydroisoxazol-3-yl)-1 -Propyl-3'H-spiro[azetidine-3,1'-isobenzofuran]-3'-one
[0541]
[0542] This compound was prepared similarly to Example 1, except that cyclopropanecarbonyl chloride was replaced with propyl chloride (10 mg, 0.1 mmol), and the alkene (1AB) was 1,3-dichloro-2-fluoro-5-( 1-trifluoromethyl-vinyl)-benzene instead of 1,2,3-trichloro-(1-trifluoromethyl-vinyl)-benzene.
[0543] The reaction was injected directly onto a 24g Redi Sep column. The crude material was chromatographed eluting with 100% heptane to 50:50 EtOAc:heptane to afford the final product (25 mg, 48%) as a solid. m / z(CI)531[M+H] + .
[0544] Preparation 9: 1-Benzhydryl-3-(2-bromo-4-(5-(3,5-dichlorophenyl-4-fluoro)-5-(trifluoromethyl)-4,5- Dihydroisoxazol-3-yl)phenyl)azetidin-3-ol
[0545]
[0546] This compound was prepared from the compound of Preparation 4 according to the method of Preparations 5 and 6 u...
Embodiment 3
[0559] Example 3: 1-(5'-(5-(3,5-dichloro-4-fluorophenyl)-5-(trifluoromethyl)-4,5-dihydroisoxazol-3-yl )-3'H-spiro[azetidine-3,1'-isobenzofuran]-1-yl)-2-((trifluoromethyl)thio)ethanone
[0560]
[0561] 5'-[5-(3,5-dichloro-4-fluorophenyl)-5-(trifluoromethyl)-4,5-dihydro-1,2-oxazol-3-yl]- 3'H-spiro[azetidine-3,1'-[2]benzofuran] (0.05mmol) was dissolved in DMF (0.5ml); it was added to [(trifluoromethyl)sulfane base] acetic acid (0.1 mmol), followed by addition of O-(7-azabenzotriazol-1-yl)-N,N,N',N'-tetramethyl-uronium hexafluorophosphate in DMF ( 0.5ml) and triethylamine (0.5mmol) in solution. The resulting mixture was shaken at ambient temperature for 16 hours. The solvent was distilled off in vacuo, and the crude product was purified by preparative HPLC to obtain 3.2 mg of the title compound. m / z[M+H] + 603; retention time 4.21 minutes.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com