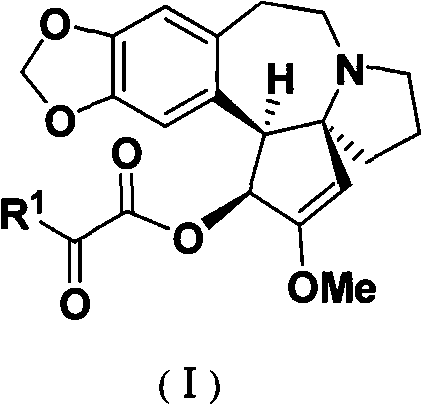
Optically pure alpha-ketoacyl harringtonine and preparing and purifying method thereof
A ketoacyl harringtonine and optical technology, applied in the direction of organic chemistry, can solve the problems of complex operation, no reported yield, difficulty of harringtonine, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1 Preparation and purification of α-ketoacyl harringtonine (general operation)
[0025] To a solution of compound (III) in dichloromethane (2 mmol, 1.0 M), 1 drop of DMF was added. Under an ice-water bath, dropwise add oxalyl chloride (2.6mmol, 1.0M) in CH 2 Cl 2 solution, remove the ice bath after the dropwise addition, the reaction stops gassing after 1.5h, continue the reaction for 1h, spin off the solvent, add benzene and spin off excess oxalyl chloride, repeat again, the residue is dissolved in 1mL of dichloromethane, drop Add harringtonine 5 (0.315 g, 1.0 mmol) and pyridine (0.22 ml, 2.6 mmol) in 2 Cl 2 solution (cooled in an ice-water bath). After the dropwise addition, the ice bath was removed. After 30 min, TLC tracked and the raw materials disappeared. Add 5 mL of water to quench the reaction, separate the layers, extract the aqueous phase three times with ether, combine the organic phases, and successively wash with 10% Na 2 CO 3 The solution w...
Embodiment 2
[0027] Example 2 Preparation of α-ketoacyl harringtonine 7
[0028]
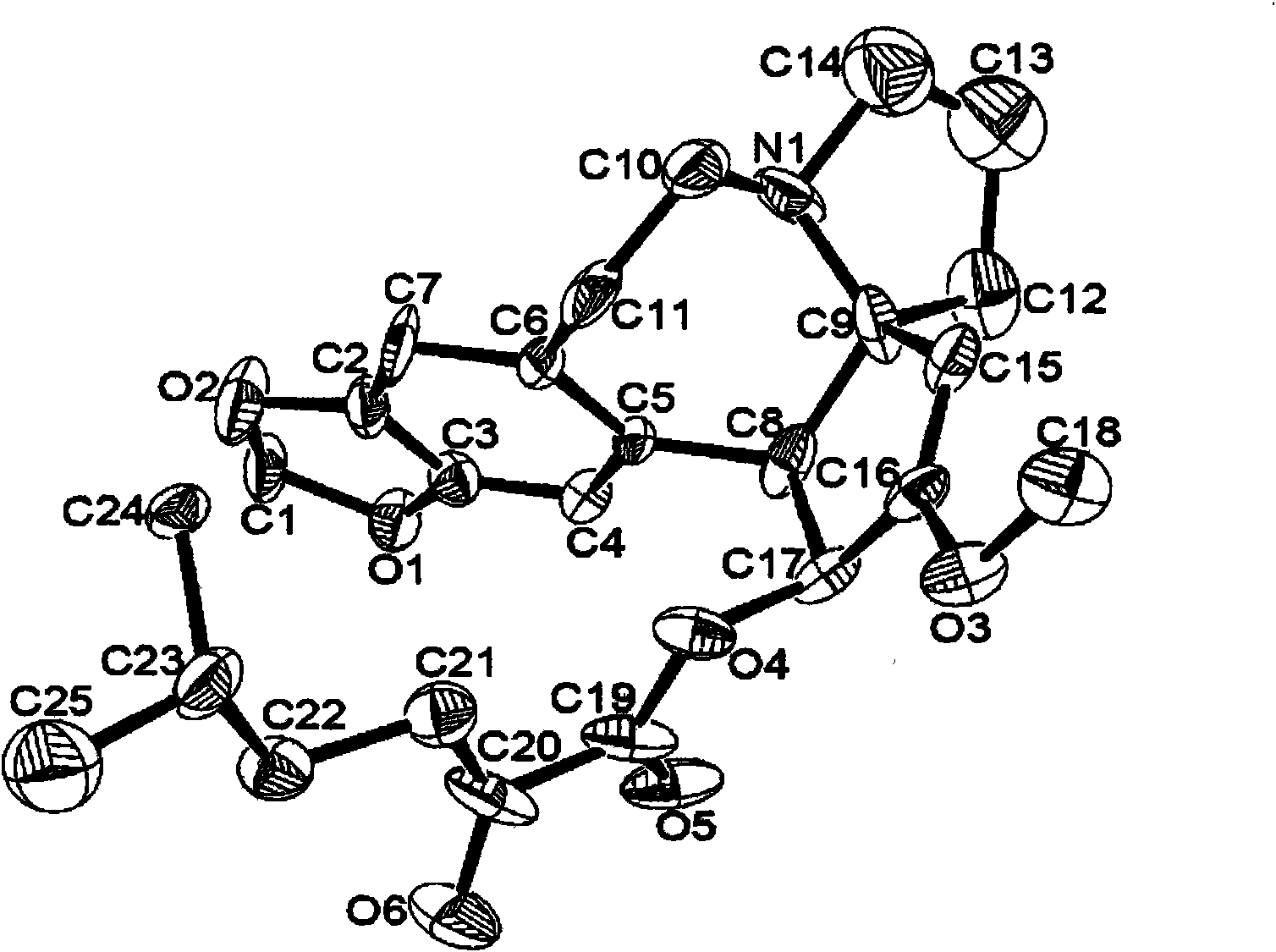
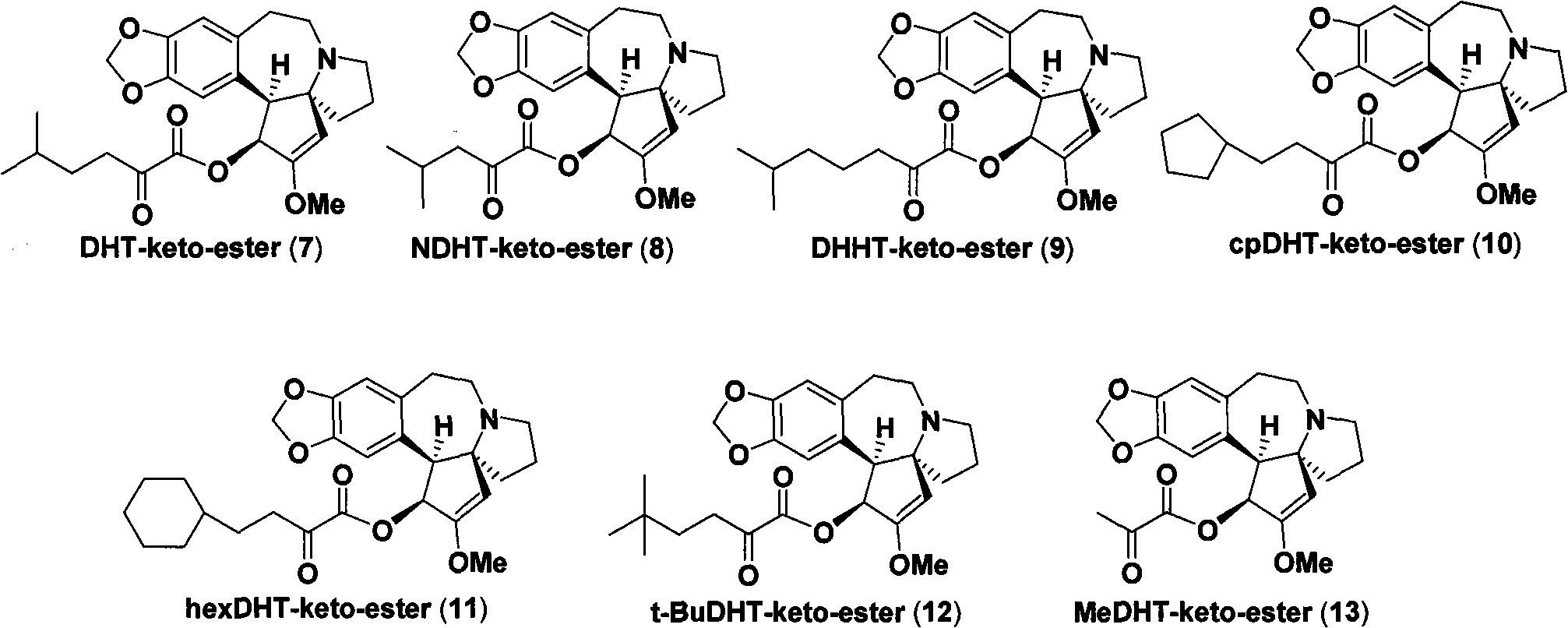
[0029] With the method of Example 1, the difference is that compound 15 is obtained with 20mmol compound 14, and then reacted with 10mmol harringtonine 5 to obtain the colorless crystal of the title compound 7 (structure as figure 1 shown). Yield 86%, mp.98~100℃; [α] D =-125.4° (c=1.0, CHCl 3 , 20℃); IR(KBr)v 2961, 2876, 1749, 1654, 937cm -1 ; 1 H NMR (400MHz, CDCl 3 )δ6.56(s, 1H, ArH), 6.54(s, 1H, ArH), 5.86(d, 1H, ArCHCH, J=9.2Hz), 5.82(s, 2H, OCH 2 O), 5.09(s, 1H, vinyl H), 3.81(d, 1H, ArCHCH, J=9.2Hz), 3.68(s, 3H, OCH 3), 3.19 (td, 1H, CH 2 , J=11.6, 7.6Hz), 3.04 (m, 1H, CH 2 ), 2.91 (m, 1H, CH 2 ), 2.57 (m, 2H, CH 2 ), 2.33 (m, 2H, CH 2 ), 2.22 (m, 1H, CH 2 ), 2.02 (dt, 1H, CH 2 , J=12.0, 9.6Hz), 1.88 (m, 1H, CH 2 ), 1.72 (m, 2H, CH 2 ), 1.41 (septet, 1H, (CH 3 ) 2 CH, J=6.8Hz), 1.24(m, 2H, CH 2 ), 0.80(d, 6H, (CH 3 ) 2 CH, J = 6.8Hz) ppm; 13 C NMR (100MHz, CDCl 3 )δ 194.1, 160....
Embodiment 3
[0030] Preparation of Example 3α-ketoacyl harringtonine 8
[0031]
[0032] The same method as in Example 1, except that 10 mmol of compound 16 was used to obtain compound 17, and then reacted with 5 mmol of harringtonine 5 to obtain colorless crystals of the title compound 8. The yield is 50%, mp.128~130℃; [α] D =-128.7° (c1.0, CHCl 3 , 20℃); IR(KBr)v 2958, 1728, 1654, 1478, 1369, 1223, 1042, 932cm -1 ; 1 H NMR (300MHz, CDCl 3 )δ6.58(s, 1H, ArH), 6.57(s, 1H, ArH), 5.88(d, 1H, ArCHCH, J=9.3Hz), 5.86(s, 1H, OCH 2 O), 5.84(s, 1H, OCH 2 O), 5.10(s, 1H, vinyl H), 3.83(d, 1H, ArCHCH, J=9.3Hz), 3.71(s, 3H, OCH 3 ), 3.20 (ddd, 1H, CH 2 , J=14.1, 12.0, 7.8Hz), 3.08 (m, 1H, CH 2 ), 2.92 (td, 1H, CH 2 , J=11.7, 7.2Hz), 2.59 (m, 2H, CH 2 ), 2.37 (dd, 1H, CH 2 , J=14.4, 6.9Hz), 2.26 (dd, 1H, CH 2 , J=16.8, 6.6Hz), 2.11 (dd, 1H, CH 2 , J=16.8, 6.9Hz), 2.04 (dt, 1H, CH 2 , J=13.2, 9.6Hz), 1.88(m, 2H, CH 2 , (CH 3 ) 2 CH), 1.75(m, 2H, CH 2 ), 0.80(d, 3H, (CH 3 ) 2 CH, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com