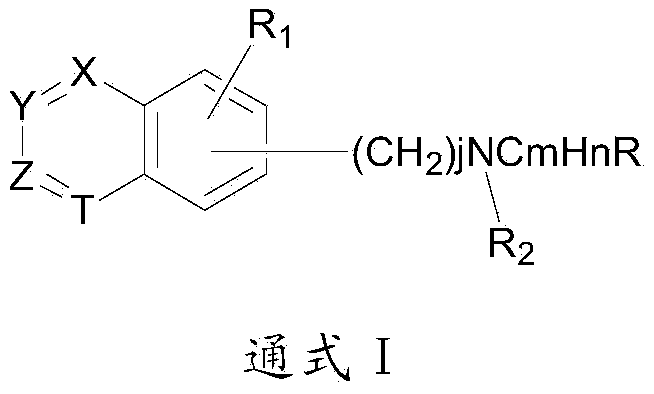
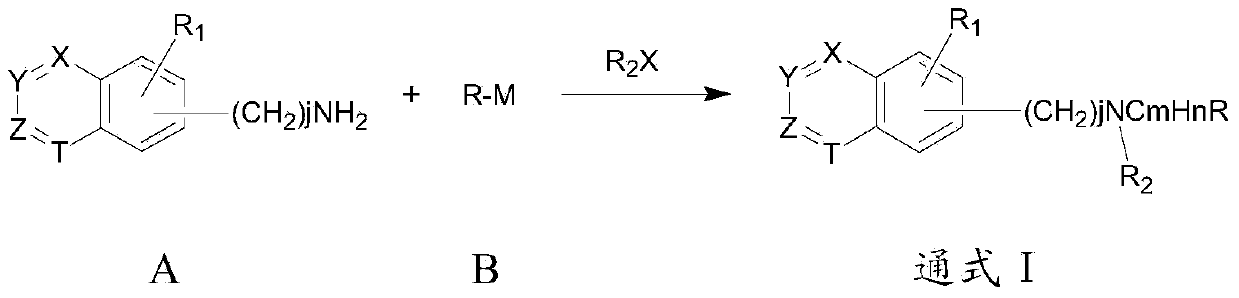
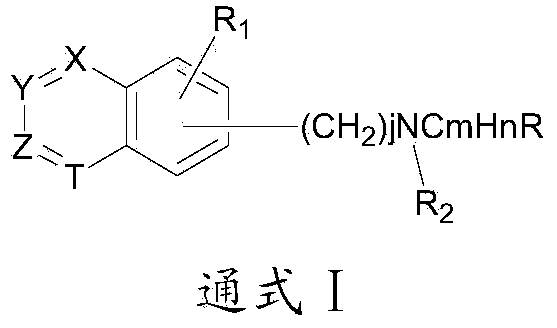
Group of substituted benzoheterocycle amine derivatives and preparation method and related application thereof as IMPDH (inosine monophosphate dehydrogenase) inhibitor
A kind of benzo, unsubstituted technology, be used in the field of substituted benzoheterocyclic amine derivatives and preparation thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] , N-(thiophene-2-methyl)-1H-indol-6-amine
[0089] Put 0.132g (0.001mol) of 6-aminoindole and 0.112 (0.001mol) of thiophene-2-carbaldehyde into a 25ml eggplant-shaped bottle, add 5ml of absolute ethanol, stir overnight at room temperature, then add 50mg of sodium borohydride, and continue the reaction for 3 Hour. After the reaction is complete, add 2ml of water, adjust the pH of the reaction solution to neutral with dilute hydrochloric acid, stir for 20 minutes, extract with dichloromethane, wash with water, wash several layers with saturated sodium chloride solution, and dry over anhydrous magnesium sulfate. After filtration and concentration, the obtained product was separated by silica gel column chromatography to obtain 0.109 g (yield: 45%) of off-white solid compound.
[0090] 1 HNMR (400MHz, DMSO-d 6 )δ (ppm): 10.47 (1H, s), 7.19 (1H, d, 8.5Hz), 6.95 (1H, d, J = 3.0Hz), 6.79 (1H, d, J = 3.0Hz), 6.60 (1H ,d,J=2.0Hz),6.50(1H,s),6.47(1H,d,J=2.0Hz),6.17(1H,s),5....
Embodiment 2
[0091] , N-(5-methylthiophene-2-methyl)-1H-indole-6-amine
[0092] Using 6-aminoindole and 5-methyl-2-thiophenecarbaldehyde as raw materials, a pale white solid compound 2 (yield: 46%) was synthesized according to the similar method of Example 1.
[0093] 1 HNMR (400MHz, DMSO-d 6 )δ (ppm): 10.45 (1H, s), 7.17 (1H, d, 8.5Hz), 6.95 (1H, d, J = 3.0Hz), 6.79 (1H, d, J = 3.0Hz), 6.60 (1H ,d,J=2.0Hz),6.47(1H,d,J=2.0Hz),6.17(1H,s),5.68(1H,t,J=6.0Hz),4.33(2H,d,6.0Hz), 2.60(3H,s)
Embodiment 3
[0094] , N-(5-chlorothiophene-2-methyl)-1H-indole-6-amine
[0095] Using 6-aminoindole and 5-chloro-2-thiophenecarbaldehyde as raw materials, a pale white solid compound 3 (yield: 47%) was synthesized according to the similar method of Example 1.
[0096] 1 HNMR (400MHz, DMSO-d 6 )δ (ppm): 10.43 (1H, s), 7.19 (1H, d, J = 8.5Hz), 6.96 (1H, d, J = 3.0Hz), 6.83 (1H, d, J = 3.0Hz), 6.71 (1H,d,J=2.0Hz),6.47(1H,d,J=2.0Hz),6.17(1H,s),5.78(1H,t,J=6.0Hz),4.34(2H,d,J= 6.0Hz);
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com