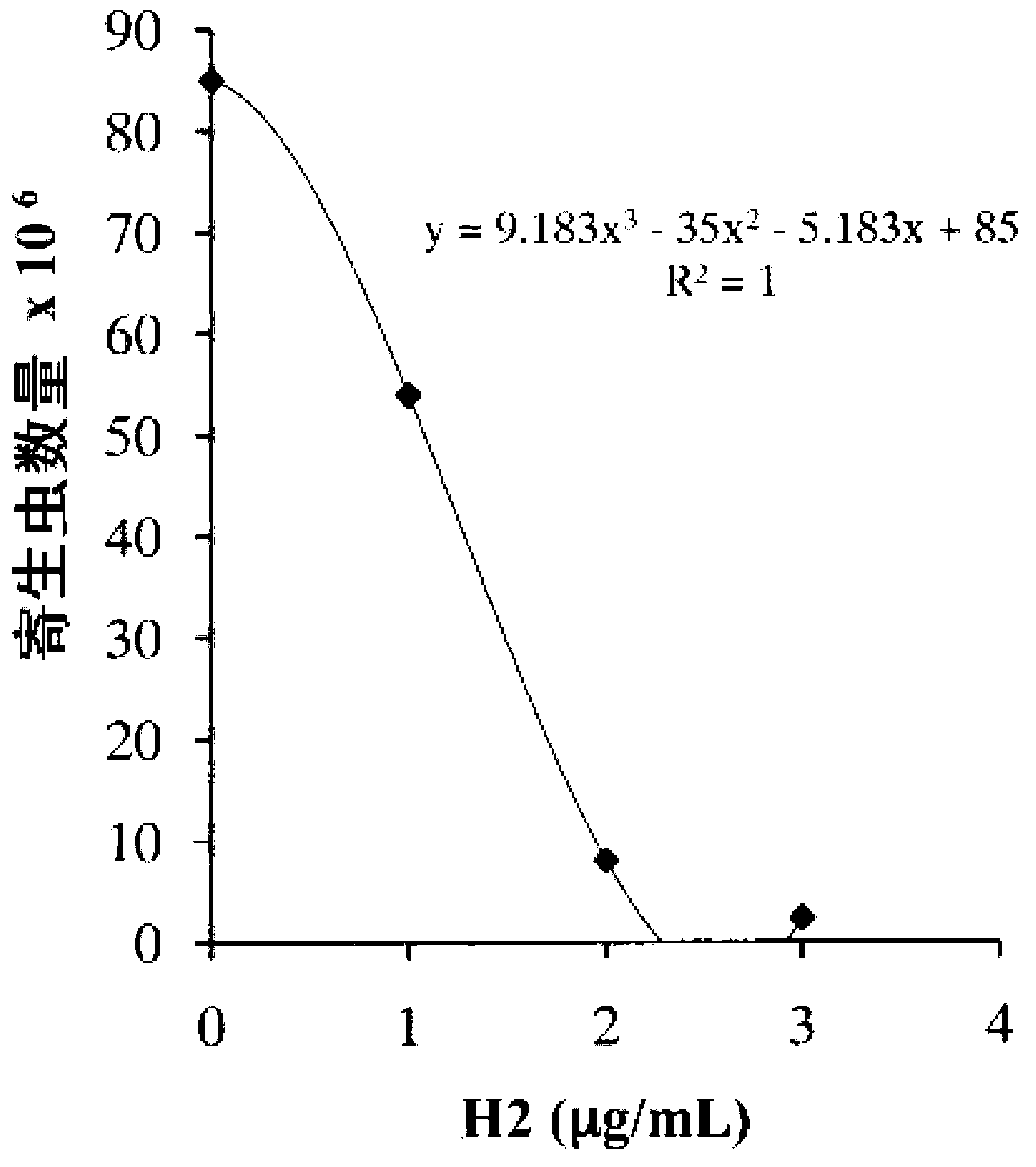
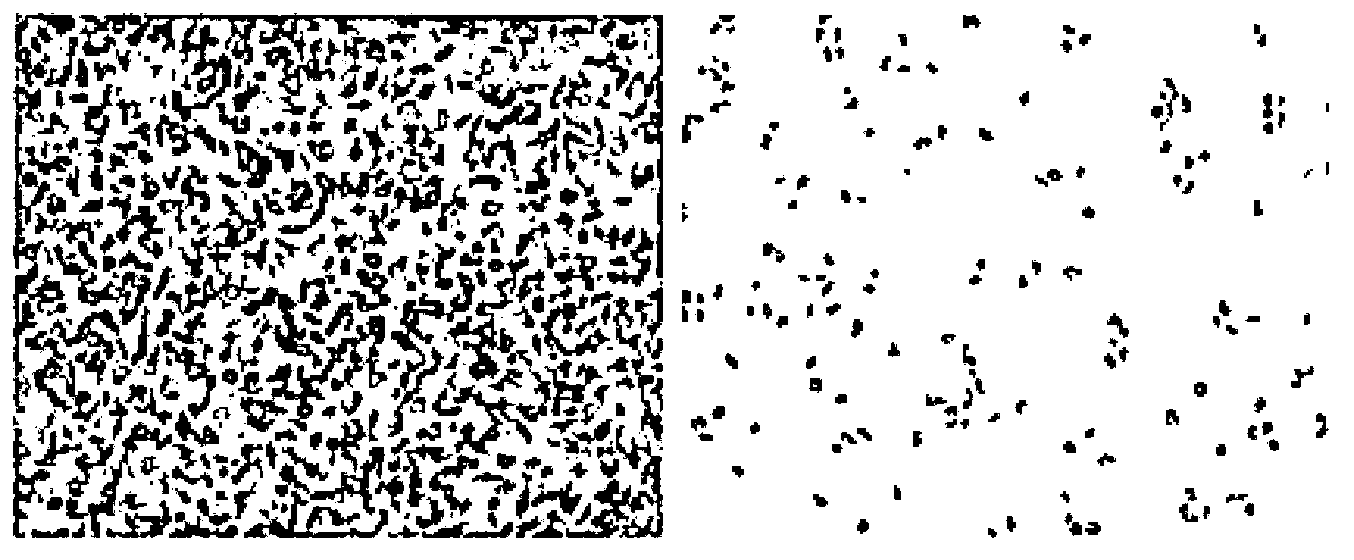
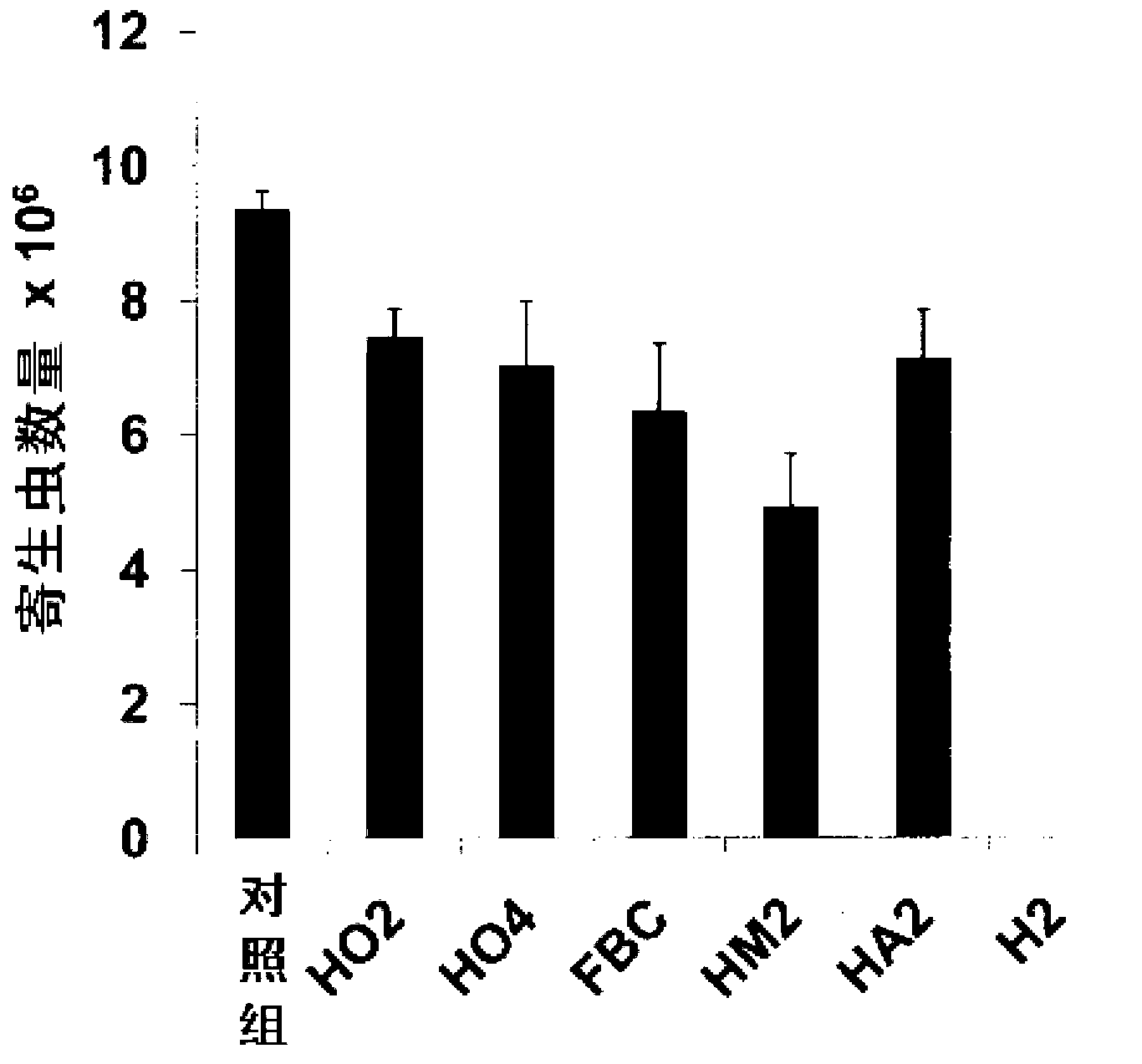
N6-(ferrocene methyl) quinazolin-2,4,6-triamine (h2) and the derivatives and prodrugs thereof as antimicrobial, antiparasitic, antiprotozoal and antileishmania agents
A technology of methyl ferrocene, derivatives, applied in the direction of iron organic compounds, resistance to vector-borne diseases, metallocenes, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0039] 1) synthesis
[0040]Using a 50ml flat-bottomed flask equipped with magnetic stirring, a Vigreux fractionating column and under a nitrogen atmosphere, 0.31g of ferrocene formaldehyde (0.00143), 0.3g of N,N'-(6-aminoquinazoline- 2,4-diyl) diacetylamine (1 equiv), 1 ml of DMF and one drop of acetic acid were mixed. The mixture was stirred at 85°C for 45 minutes. The mixture was then cooled to 0°C using an ice-water bath, and 0.0671 g (2 equiv) of NaBH was added slowly 4 . The ice bath was removed and stirring was continued at room temperature for 12 hours. DMF was evaporated in a rotary evaporator, and Na 2 CO 3 Saturated solution was added to the residue. The yellow precipitate formed was isolated by filtration and washed several times with water. After drying at room temperature, the solid was washed several times with diisopropyl ether to afford 0.3239 g of HA in 48% yield, R f =0.76(CHCl 3 / MeOH80:20), p.f.=218–220°C. HA2 was hydrolyzed with one normal metha...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com