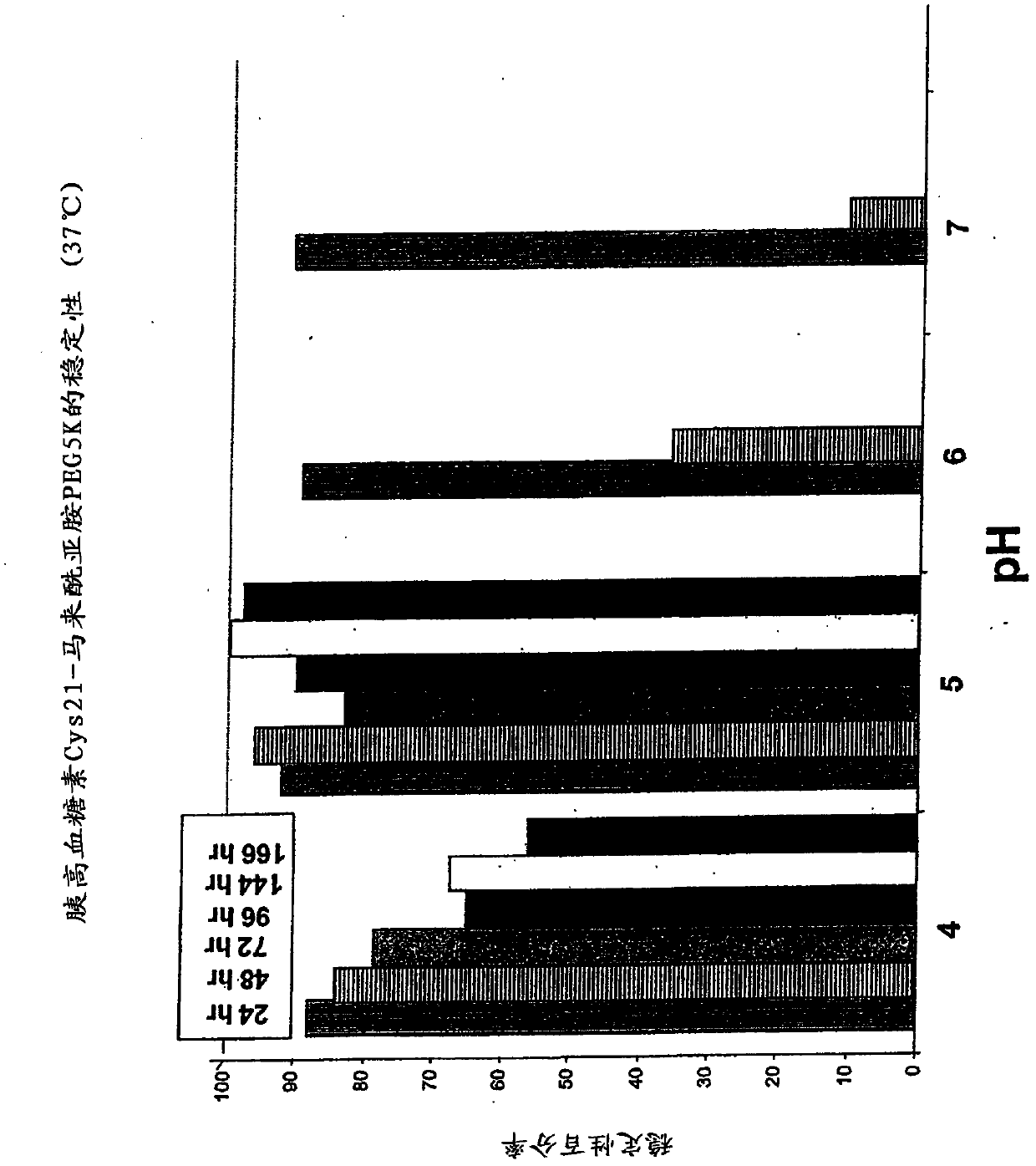
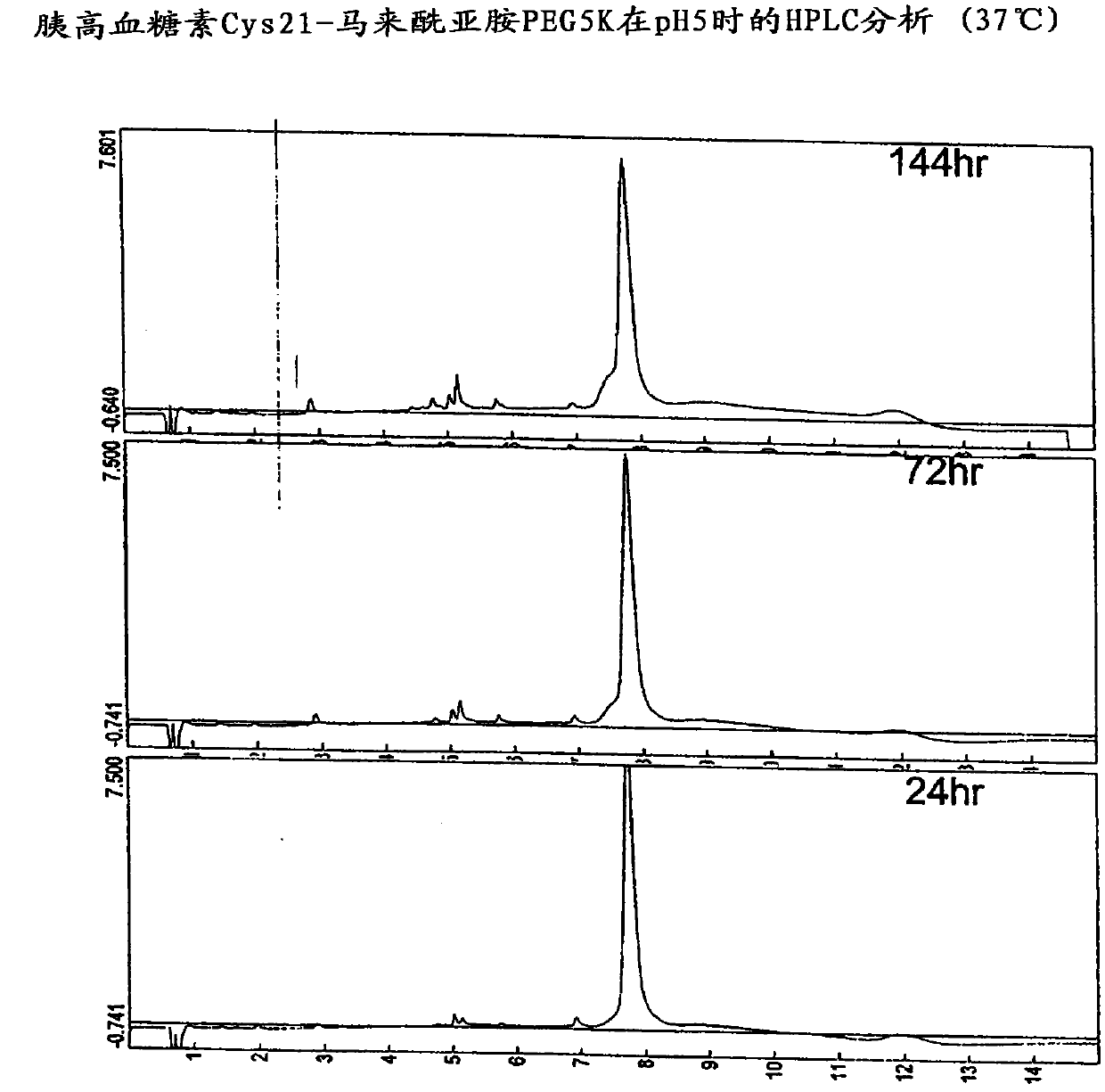
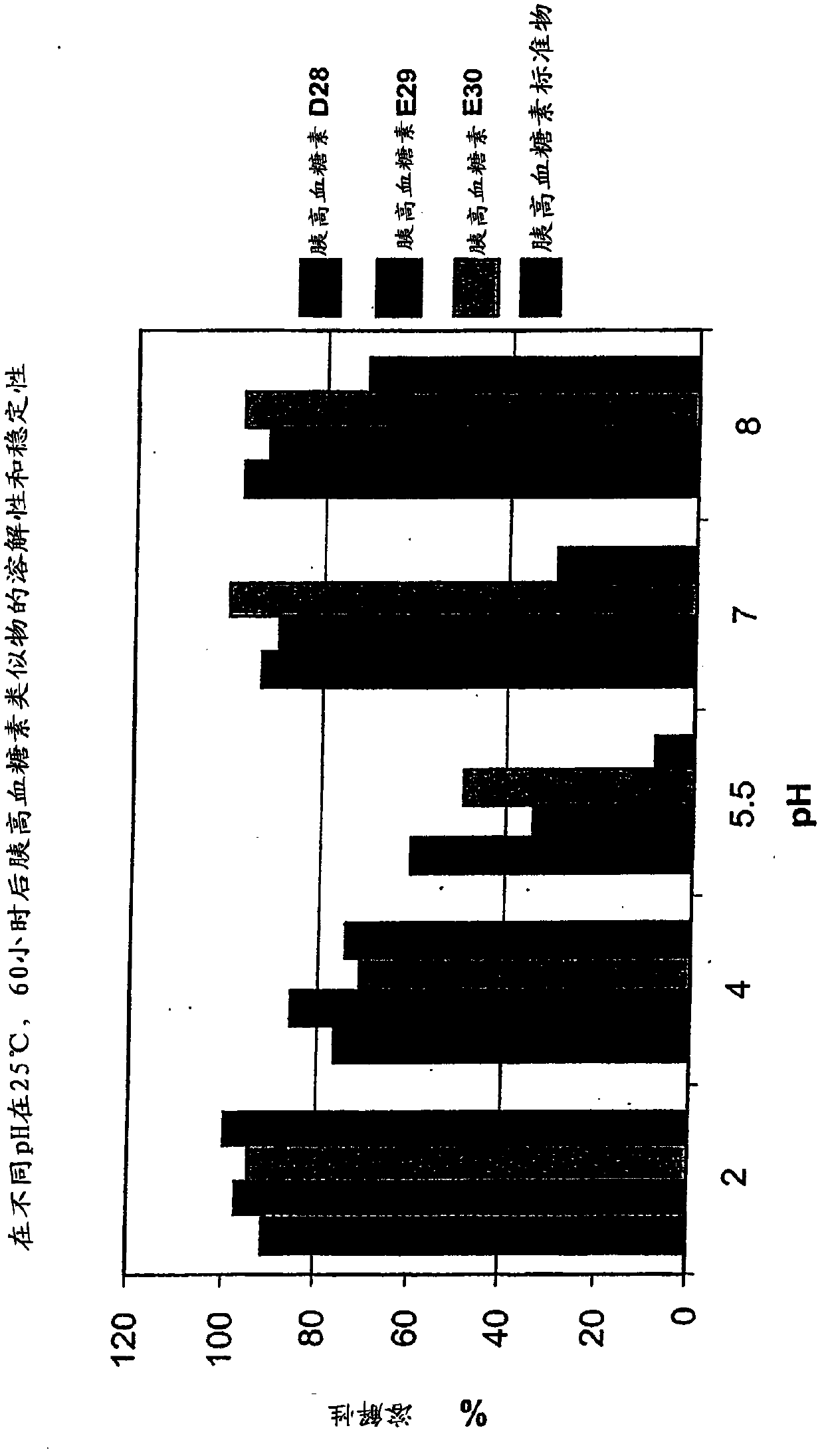
Glucagon analogs exhibiting enhanced solubility and stability physiological pH buffers
A technology of glucagon and glucagon peptide, applied in the field of glucagon analogs with enhanced solubility and stability in a physiological pH buffer, can solve problems such as troublesome methods, dangerous personnel and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0124] enhanced solubility
[0125] Applicants have discovered that native glucagon can be modified by introducing charges at its carboxyl terminus to enhance the solubility of the peptide while maintaining the agonist properties of the peptide. The enhanced solubility allows preparation and storage of glucagon solutions at near neutral pH. Formulating the glucagon solution at a relatively neutral pH (eg, a pH of about 6.0 to about 8.0) improves the long-term stability of the glucagon peptide.
[0126] Accordingly, one embodiment of the present invention relates to a glucagon agonist which, relative to the wild-type peptide His-Ser-Gln-Gly-Thr-Phe-Thr-Ser-Asp-Tyr-Ser-Lys-Tyr- Leu-Asp-Ser-Arg-Arg-Ala-Gln-Asp-Phe-Val-Gln-Trp-Leu-Met-Asn-Thr (SEQ ID NO: 1) has been modified to improve the peptide in aqueous solution Solubility, especially in the pH range of about 5.5 to about 8.0, while retaining the biological activity of the original peptide. In one embodiment, the charge is...
example
[0321] According to one embodiment, the original glucagon peptide of SEQ ID NO: 1 is modified by replacing the original amino acid at position 28 and / or 29 with a negatively charged amino acid (such as aspartic acid or glutamic acid), and optionally A negatively charged amino acid (eg, aspartic acid or glutamic acid) is optionally added to the carboxy-terminus of the peptide. In an alternative embodiment, the original glucagon peptide of SEQ ID NO: 1 is modified by replacing the original amino acid at position 29 with a positively charged amino acid (eg lysine, arginine or histidine) , and optionally add 1 or 2 positively charged amino acids (eg, lysine, arginine, or histidine) to the carboxyl terminus of the peptide. According to one embodiment, there is provided a glucagon analog with improved solubility and stability, wherein said analog comprises the amino acid sequence of SEQ ID NO: 34, with the proviso that at least one amino acid at position 28 or 29 is replaced by aci...
Embodiment 1
[0392] Glucagon Cys 17 Synthesis of (1-29) and similar MonoCys analogs
[0393] Synthesis was performed using FastBoc HBTU-activated single coupling on a modified Applied Biosystem 430A peptide synthesizer inputting 0.2 mmole Boc Thr(OBzl) Pam resin (SynChem Inc) in a 60 ml reaction tube and the following sequence.
[0394] HSQGTFTSDYSKYLDSCRAQDFVQWLMNT (SEQ ID NO: 27)
[0395] The following side chain protecting groups were used: Arg(Tos), Asp(OcHex), Asn(Xan), Cys(pMeBzl), Glu(OcHex), His(Boc), Lys(2Cl-Z), Ser(Bzl), Thr (Bzl), Trp (CHO) and Tyr (Br-Z). The completed peptidyl resin was treated with 20% piperidine / dimethylformamide to remove the Trp formyl protection, then transferred to an HF reaction tube and dried in vacuo. Add 1.0 ml p-cresol and 0.5 ml dimethyl sulfide and a magnetic stir bar. The tube was connected to HF equipment (Pennisula Labs), cooled in a dry ice / methanol bath, evacuated, and condensed into approximately 10 ml of liquid hydrogen fluoride. The r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com