Endomorphin-1 analogue modified by alpha-alkenyl-beta-amino acid and composition and application thereof
An endomorphin and amino acid technology, applied in the field of synthetic methods, can solve the problems such as inability to obtain better effects, and achieve the effects of good pharmacological activity, improved bioavailability, and increased resistance to enzymatic hydrolysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
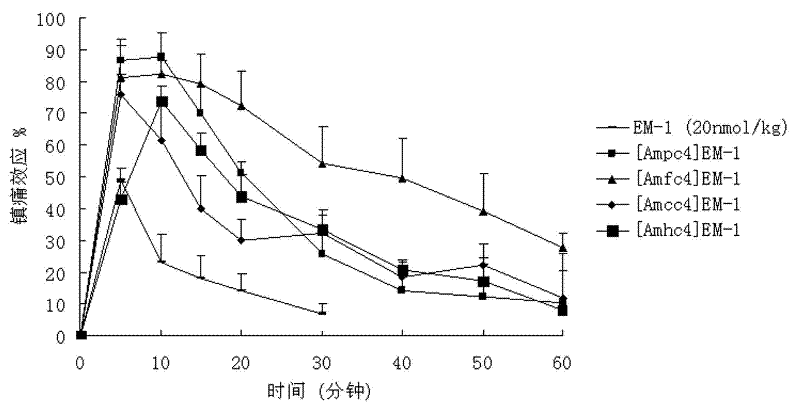
[0100] Embodiment one, [Amp 4 ] Synthesis of EM-1
[0101] 1. Synthesis of 3-amino-2-methenyl-3-phenylpropionic acid
[0102] (1) Dimethyl 2-(N-acylpyrrole)ethylphosphonate 1.5 moles and 1-(3,5-bistrifluoromethyl)-3-((1S,2S)-2(1-pyrrole Dissolve 0.2 moles of alkane)cyclohexyl)thiourea catalyst in toluene, stir at 0°C, add 1 mole of N-tert-butoxycarbonylbenzenesulfonylphenylimine and 0.8 liter of potassium carbonate solution, and react at 0°C for 24 Hour, after TLC detection reaction finishes, silica gel column chromatography separates and purifies (PE:EA=1:1, R f =0.4), to obtain colorless oily product (2-(dimethoxyphosphoryl)-3-carbonyl-1-phenyl-3-(1-pyrrole) propyl) tert-butyl carbamate, yield 81% .
[0103](2) Dissolve 0.8 moles of the compound (2-(dimethoxyphosphoryl)-3-carbonyl-1-phenyl-3-(1-pyrrole) propyl) tert-butyl carbamate in anhydrous tetrahydrofuran at minus Add 1.76 moles of sodium methoxide solution dissolved in methanol at 18°C, add 4 moles of paraformald...
Embodiment 2
[0126] Embodiment two, [Amf 4 ] Preparation of EM-1
[0127] 1. Synthesis of 3-amino-2-methenyl-3-(2-furan)propionic acid
[0128] (1) Dimethyl 2-(N-acylpyrrole)ethylphosphonate 1.5 moles and 1-(3,5-bistrifluoromethyl)-3-((1S,2S)-2(1-pyrrole Dissolve 0.2 moles of alkane) cyclohexyl) thiourea catalyst in toluene, stir at 0°C, add 1 mole of N-tert-butoxycarbonylbenzenesulfonyl 2-furimide and 0.8 liter of potassium carbonate solution, and react at 0°C After 24 hours, after the reaction was detected by thin plate chromatography, silica gel column chromatography was used to separate and purify (PE:EA=1:1, R f =0.4), a colorless oily product (tert-butyl 2-(dimethoxyphosphoryl)-3-carbonyl-1-furan-3-(1-pyrrole)propyl)carbamate was obtained with a yield of 85%.
[0129] (2) The compound (2-(dimethoxyphosphoryl)-3-carbonyl-1-furan-3-(1-pyrrole)propyl) tert-butyl carbamate 0.8 mol dissolved in anhydrous tetrahydrofuran, minus 18°C Add 1.76 moles of sodium methoxide solution dissolve...
Embodiment 3
[0153] Embodiment three, [Amc 4 Synthesis of ]EM-1
[0154] 1. Synthesis of 3-amino-2-methenyl-3-(3-chlorophenyl)propionic acid:
[0155] (1) Dimethyl 2-(N-acylpyrrole)ethylphosphonate 1.5 moles and 1-(3,5-bistrifluoromethyl)-3-((1S,2S)-2(1-pyrrole Dissolve 0.2 moles of alkane)cyclohexyl)thiourea catalyst in toluene, stir at 0°C, add 1 mole of N-tert-butoxycarbonylbenzenesulfonyl 3-chlorophenylimine and 0.8 liters of potassium carbonate solution, at 0°C The reaction was carried out for 24 hours, and after the reaction was detected by thin plate chromatography, silica gel column chromatography was used for separation and purification (PE:EA=1:1, R f =0.4), a colorless oily product—(2-(dimethoxyphosphoryl)-3-carbonyl-1-(3-chlorophenyl)-3-(1-pyrrole) propyl) carbamic acid tert Butyl ester, yield 91%.
[0156] (2) The compound (2-(dimethoxyphosphoryl)-3-carbonyl-1-(3-chlorophenyl)-3-(1-pyrrole)propyl) tert-butyl carbamate 0.8 mole dissolved in Water tetrahydrofuran, add 1.76...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com