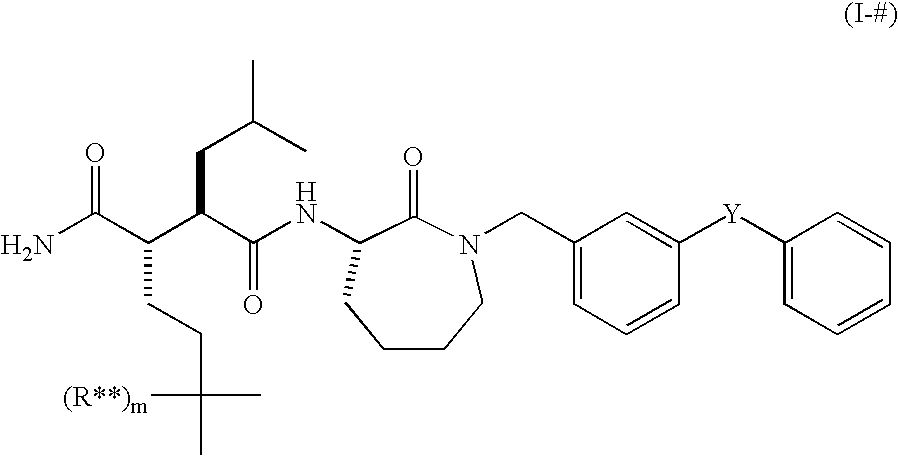
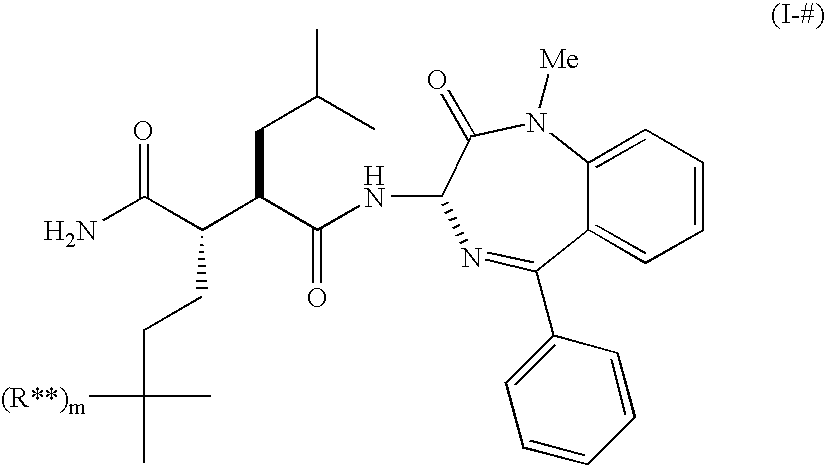
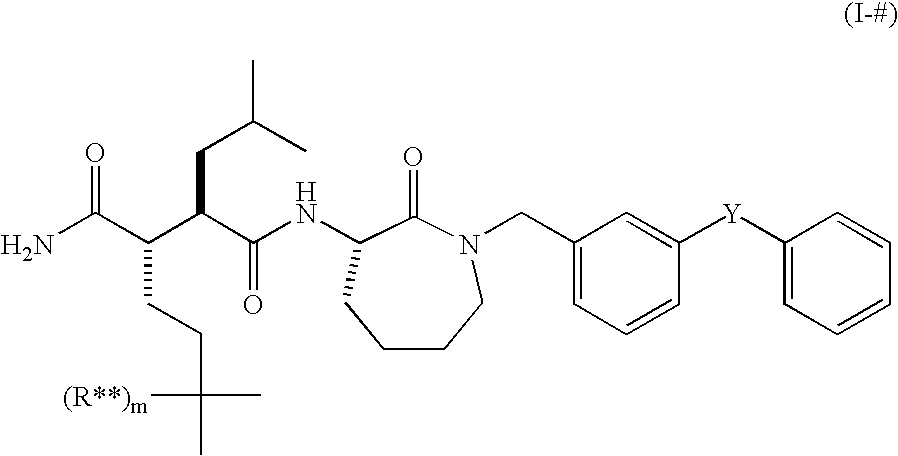
Use of small molecule radioligands to discover inhibitors of amyloid-beta peptide production and for diagnostic imaging
a technology of amyloidbeta peptide and small molecule radioligand, which is applied in the direction of radioactive preparation carriers, in-vivo testing preparations, therapy, etc., can solve the problems of major present and future health problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
(2R,3S) N1-[(3S)-hexahydro-1-(3,3-diphenylpropyl)-2-oxo-1H-azepin-3-yl]-N-4-(hydroxy)-2-(2-methylpropyl)-3-(propyl)-butanediamide
[0716]
[0717] Step (1a): Di-tert-butyldicarbonate (10.2 g, 46.7 mmoles) was added portion wise to a solution of L-(−)-α-amino-ε-caprolactam (5.0 g, 39.0 mmoles) in dimethyl sulfoxide (30 mL). After 5 h at rt, the reaction was partitioned between water (100 mL) and ethyl acetate. The combined organic extracts were washed successively with 1 M HCl (50 mL), brine, and dried (MgSO4) and concentrated in vacuo. The residue was recrystallized in 1:1 v / v ether-hexanes, two crops yielded the desired product (6.26 g, 70%) as white solid. MS (M+H-BOC)+=129.
[0718] Step (1b): Triphenylphosphine (3.0 g, 11.4 mmoles) and carbon tetrabromide (3.75 g, 11.7 mmoles) were added successively to a cooled (0° C.) solution of 3,3-biphenyl-1-propanol (1.5 mL, 7.5 mmoles) in dichloromethane (20 mL). After 1.5 hours at rt, the mixture was concentrated in vacuo. The residue was puri...
example 2
(2R,3S) N1-[(3S)-hexahydro-1-(3-phenoxybenzyl)-2-oxo-1H-azepin-3-yl]-N-4-(hydroxy)-2-(2-methylpropyl)-3-(propyl)-butanediamide
[0724]
[0725] Step (2a): Triphenylphosphine (3.40 g, 13.0 mmoles) and carbontetrabromide (4.20 g, 13.0 mmoles) were added successively to a solution of m-phenoxybenzyl alcohol (1.5 mL, 8.6 mmoles). After 4 h at rt the mixture was concentrated and was purified by silica gel column (hexanes, then ethyl acetate:hexanes, 5:95) to give the desired bromide (1.3 g, 57%) as a yellow oil. MS (M-Br)+=183.
[0726] Step (2b): A 1 M solution of lithium bis(trimethylsilyl)amide was added dropwise to a solution of compound of Step (1a) (0.3 g, 1.31 mmoles) in tetrahydrofuran (5 mL) at −78° C. After 30 minutes a solution of compound of Step (2a) (0.43 g, 1.63 mmoles) in tetrahydrofuran (4 mL) was added to the mixture dropwise. The reaction was allowed to come to ambient temperature, stirred for 16 h, then partitioned between water and ethyl acetate. The combined organic extra...
example 3
(2R,3S) N1-[(3S)-hexahydro-1-(phenyl)-2-oxo-1H-azepin-3-yl]-N-4-(hydroxy)-2-(2-methylpropyl)-3-(propyl)-butanediamide
[0731]
[0732] Step (3a): Triethylamine (1.5 mL, 10.8 mmoles), copper (II) acetate (0.95 g, 5.2 mmoles) and phenylboric acid (1.6 g, 13.1 mmoles) were added successively to a solution of compound of Step (1a) (1.0 g, 4.4 mmoles) in dichloromethane (20 mL). After 2.5 h at rt, more phenylboric acid (0.5 g, 4.1 mmoles) was added to the mixture. After an additional 3 hours at rt more phenylboric acid (0.5 g, 4.1 mmoles) was added to the mixture. After 65 h at rt, the mixture was filtered over celite. The filtrate was concentrated in vacuo, and the residue was purified by silica gel chromatography (ethyl acetate:hexanes, 5:95 then 15:85) to give the desired product (250 mg, 19%). MS (M-Ot-Bu)+=231.
[0733] Step (3b): Following a procedure analogous to the preparation of compound of Step (2c), but using compound of Step (3a) (250 mg, 0.82 mmoles), the amine (300 mg, 99%) was ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
| Electric charge | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com