Patents
Literature
37results about How to "Reduce proliferation rate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Gelatin sponge and preparation method thereof
InactiveCN101574539AImprove performancePromote absorptionSurgeryAbsorbent padsGelatin spongeIrradiation
The invention provides gelatin sponge, gelatin aqueous solution is irradiated to crosslink to form gelatin hydrogel, and then the gelatin hydrogel is swollen, frozen and dried to obtain the gelatin sponge. The invention also provides a preparation method of the gelatin sponge by an irradiation crosslinking way, the whole process flow is finished in a pure water system, the irradiation and crosslinking processes have sterilizing function and good controllability, the obtained product has better structural uniformity compared with a chemical crosslinking method, and the gelatin sponge has unique advantages as biomedical materials.
Owner:厦门凝赋生物科技有限公司
Method for inducing adventitious buds by adopting Lycoris radiate rachis as explant
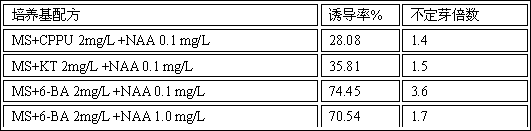
ActiveCN106561450AReduce reproductionGet efficientlyHorticulture methodsPlant tissue cultureBudInflorescence
The invention provides a method for inducing adventitious buds by adopting Lycoris radiate rachis as explant in order to obtain regenerated plants. The method comprises the following steps: selection and disinfection of the explant, induction of the adventitious buds, subculture and propagation, root induction, and transplantation of tissue culture plants. The method allows the induction germination rate to reach 96%, 4.9 times or above adventitious buds to be proliferated from a per plant and the rooting rate to reach 94%, and can effectively solve the problem of in vitro rapid propagation of Lycoris radiate.
Owner:INST OF BOTANY JIANGSU PROVINCE & CHINESE ACADEMY OF SCI
Generation of tumor-free embryonic stem-like pluripotent cells using inducible recombinant rna agents
ActiveCN101970664AIncrease translation efficiencyStrong gene silencing effectGenetically modified cellsCell culture active agentsCancer cellMammal
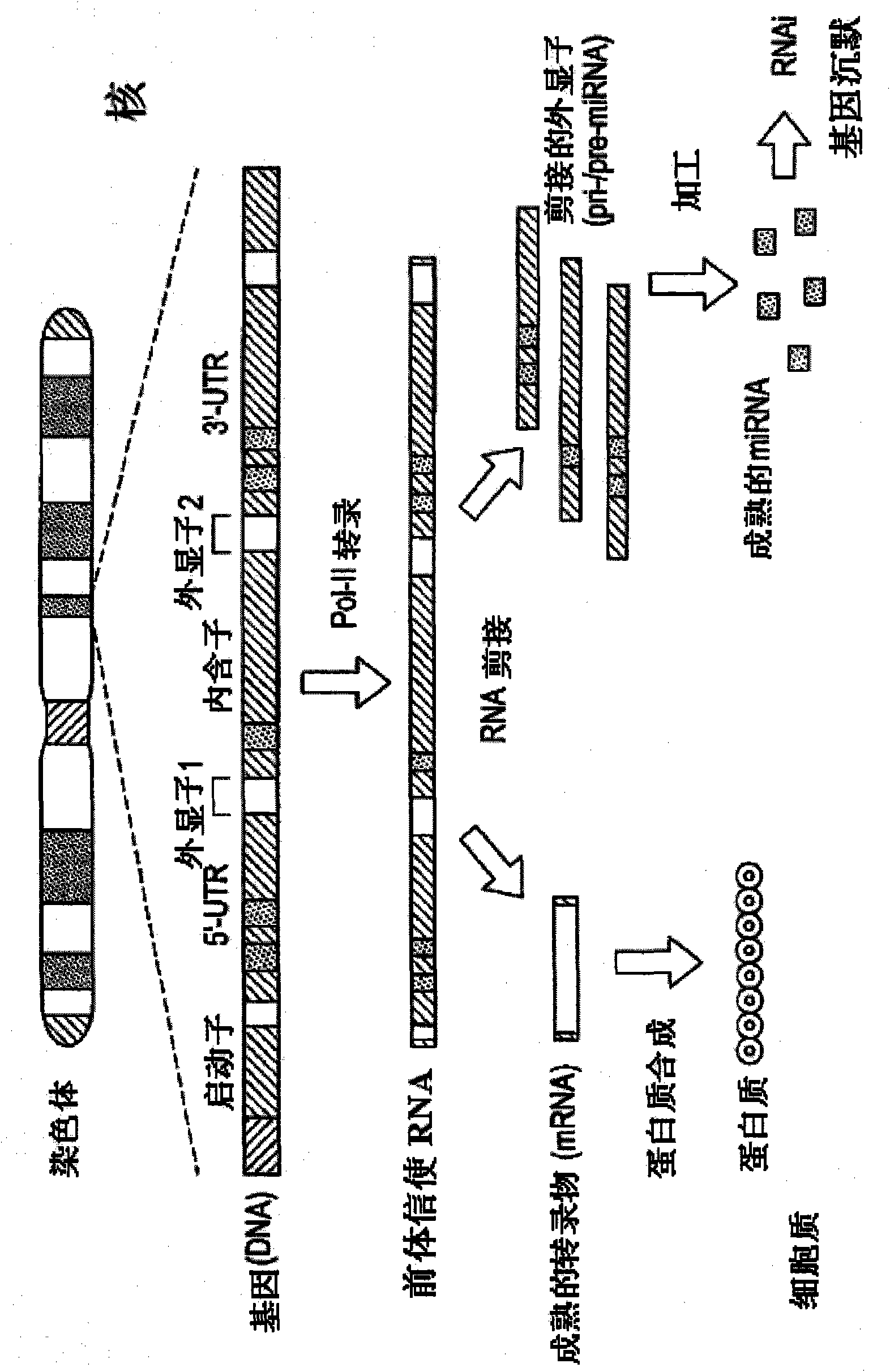
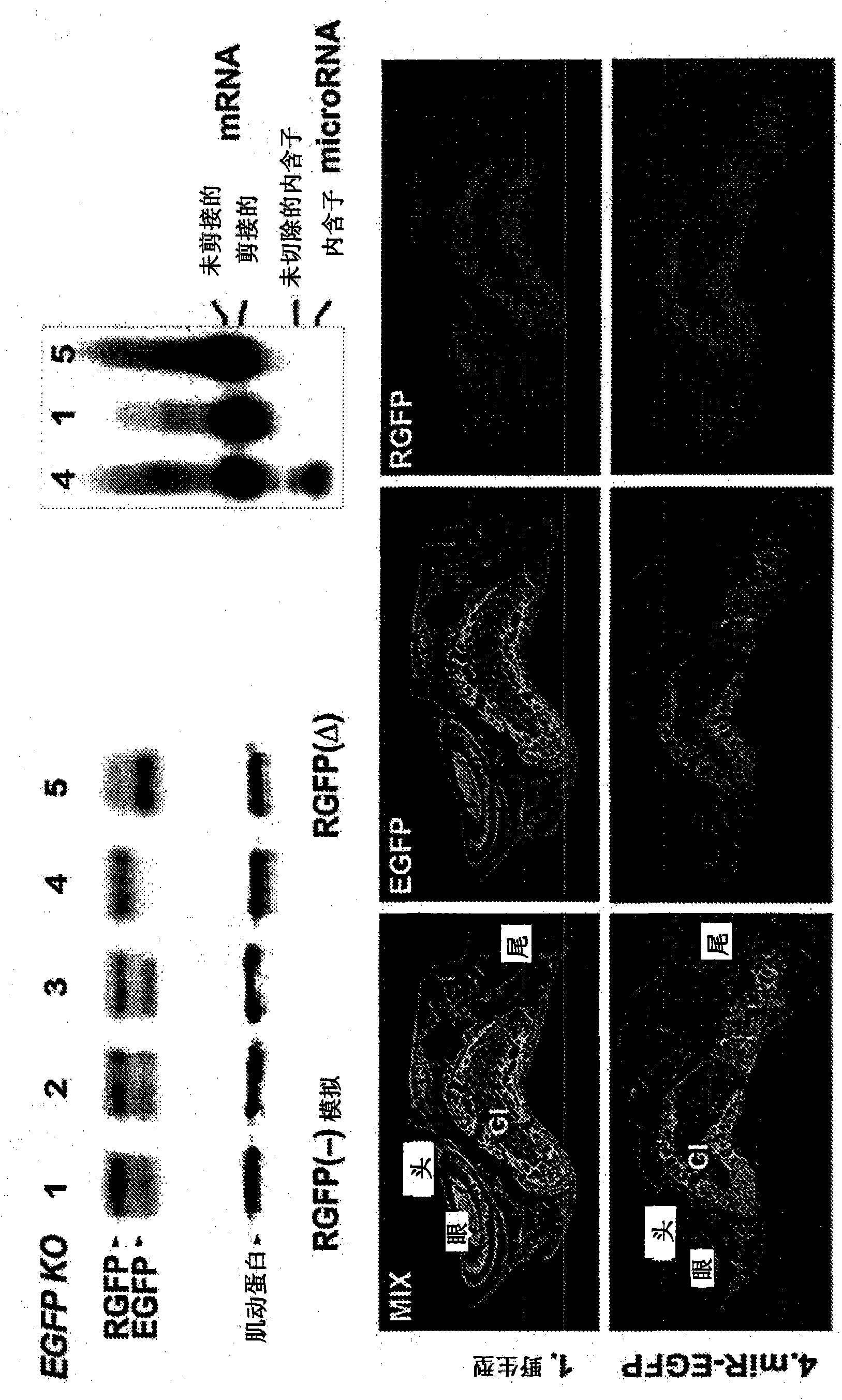
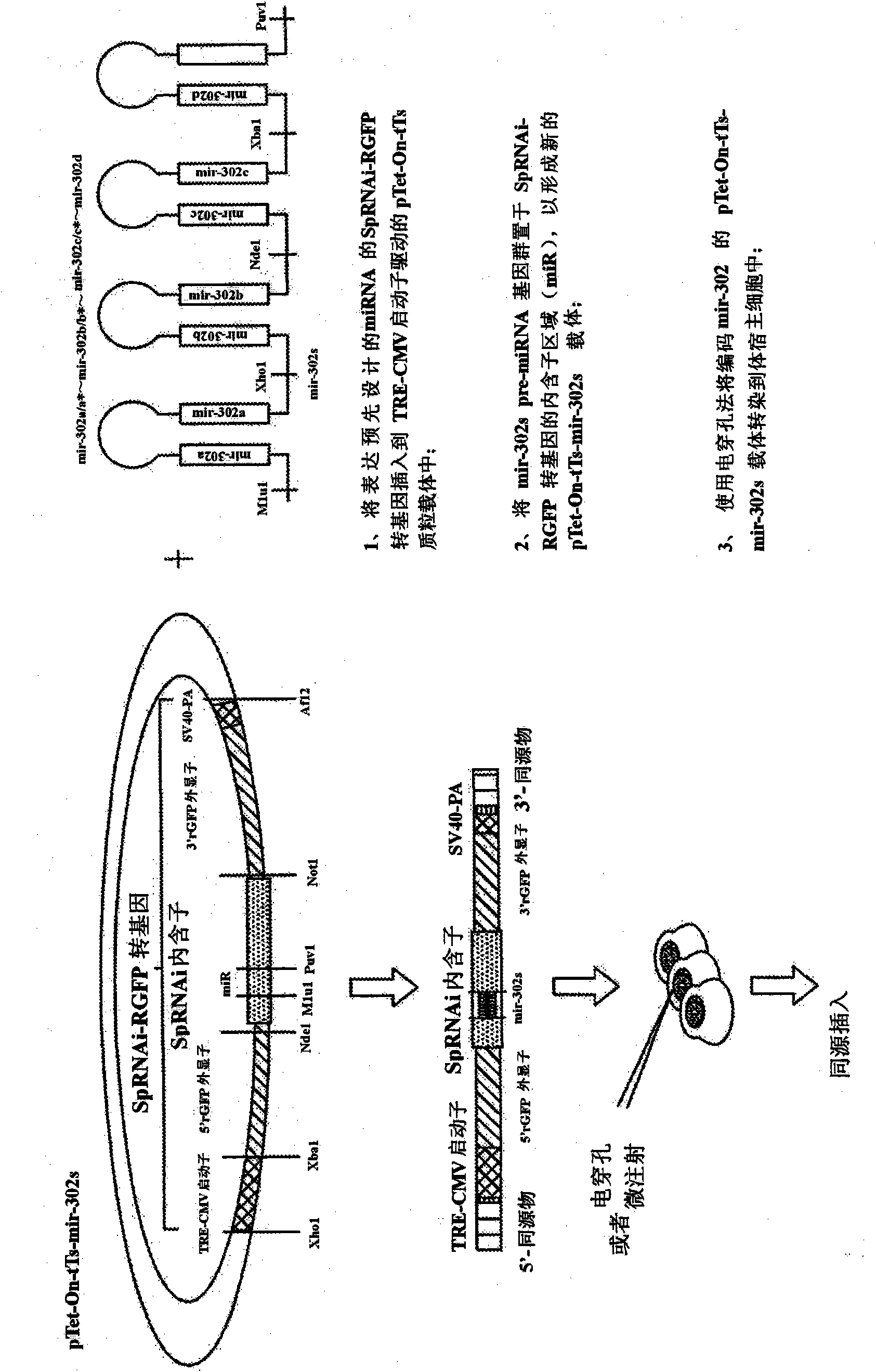
The present invention generally relates to a method for developing, generating and selecting tumor-free embryonic stem (ES)-like pluripotent cells using electroporation delivery of an inducible tumor suppressor mir-302 agent into mammalian cells. More particularly, the present invention relates to a method and composition for generating a Tet-On / Off recombinant transgene capable of expressing a manually re-designed mir-302 microRNA (miRNA) / shRNA agent under the control of doxycyclin (Dox) in human somatic / cancer cells and thus inducing certain specific gene silencing effects on the differentiation-associated genes and oncogenes of the cells, resulting in reprogramming the cells into an ES-like pluripotent state.
Owner:林希龙 +1
Tissue culture method for obtaining regenerated plantlet by using hippeastrum vittatum plateau as explant
InactiveCN110447537AReduce proliferation rateEfficient reproductionHorticulture methodsPlant tissue cultureBudPlateau
The invention provides a tissue culture method for obtaining a regenerated plantlet by using a hippeastrum vittatum plateau as an explant. The method comprises the following steps that firstly, explant selection is conducted, and then disinfection is conducted; after disinfection, a bulb is cut into 8-12 blocks according to the size and then inoculated into an induction culture medium for adventitious bud induction; the culture temperature is controlled to be 24-26 DEG C, and under the condition that the illumination time is 10-14 hours / day, and the illumination intensity is 2000-2200 lx, normal culture is conducted for 45 days, and leaves and roots are removed from bulblets obtained through proliferation, and the seedlings obtained through adventitious bud development are cut and transferred into a subculture medium for culture for 25-35 days, and finally, the aseptic seedlings are inoculated into a rooting culture medium for rooting culture for 15-25 days. Through the method, the problem about rapid in-vitro propagation of a new hippeastrum vittatum variety can be effectively solved.
Owner:INST OF BOTANY JIANGSU PROVINCE & CHINESE ACADEMY OF SCI
Chinese medicine composition for removing scars and preparation method thereof
ActiveCN103251707AReasonable useEasy to useAnthropod material medical ingredientsDermatological disorderCutaneous microcirculationSkin elasticity
The invention belongs to the field of medicines, and in particular relates to a Chinese medicine composition for removing scars and a preparation method thereof. The Chinese medicine composition is prepared from the following raw materials in parts by weight: 6-12 parts of radix angelicae, 6-12 parts of the root of red-rooted salvia, 4-8 parts of centipede, 5-10 parts of dandelion, 3-8 parts of safflower carthamus, 5-10 parts of sanguisorba officinalis, 5-10 parts of pearl powder, 1-4 parts of camphor, 4-10 parts of honey and 8-15 parts of Vaseline. The Chinese medicine composition has the advantages of stable effect, rapid efficacy, fast acting, short treatment course and significant effect, and is convenient to use; and meanwhile the Chinese medicine composition has the functions in improving skin microcirculation of a wounded part, recovering the skin elasticity of the wounded part, removing pigmentation, activating blood and removing scars, detoxifying and covering, prompting granulation and removing stasis, moistening skin and the like; and furthermore, the Chinese medicine composition can be used for treating scars of various types.
Owner:郑州密丽药业有限公司
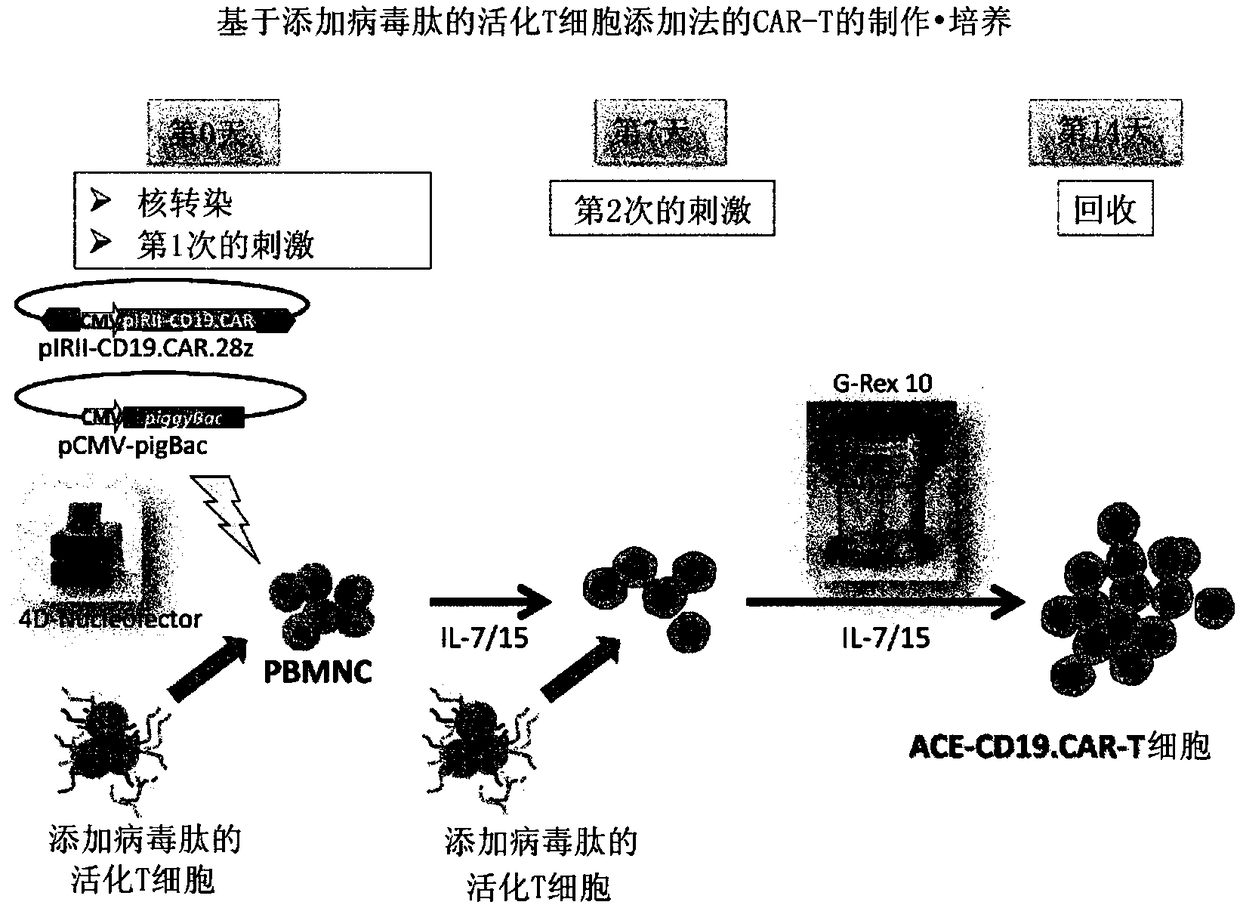
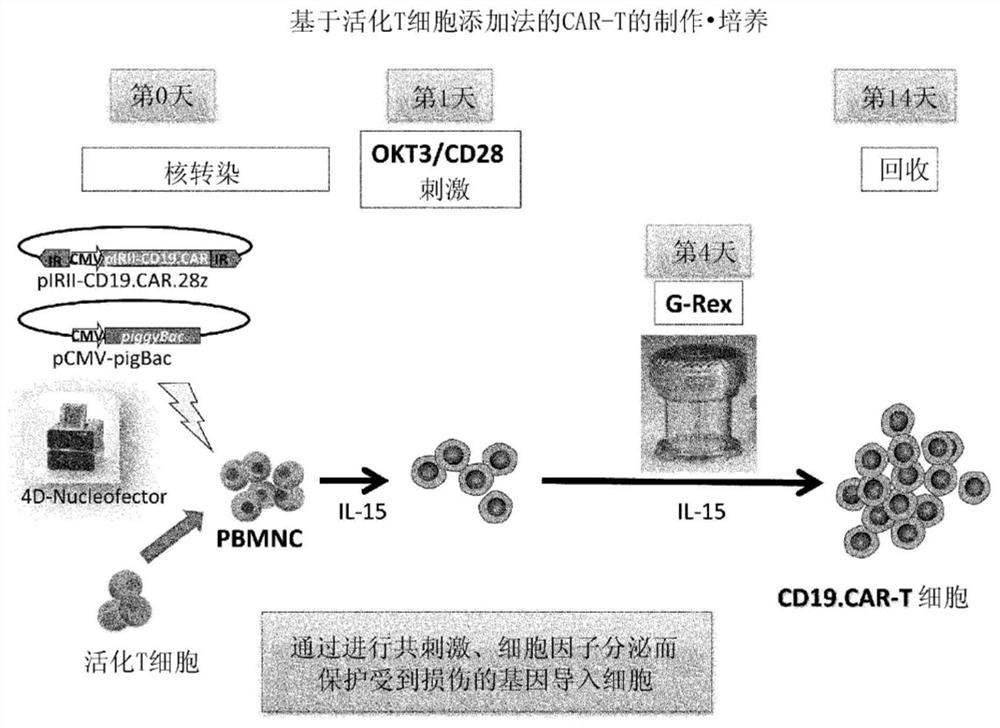
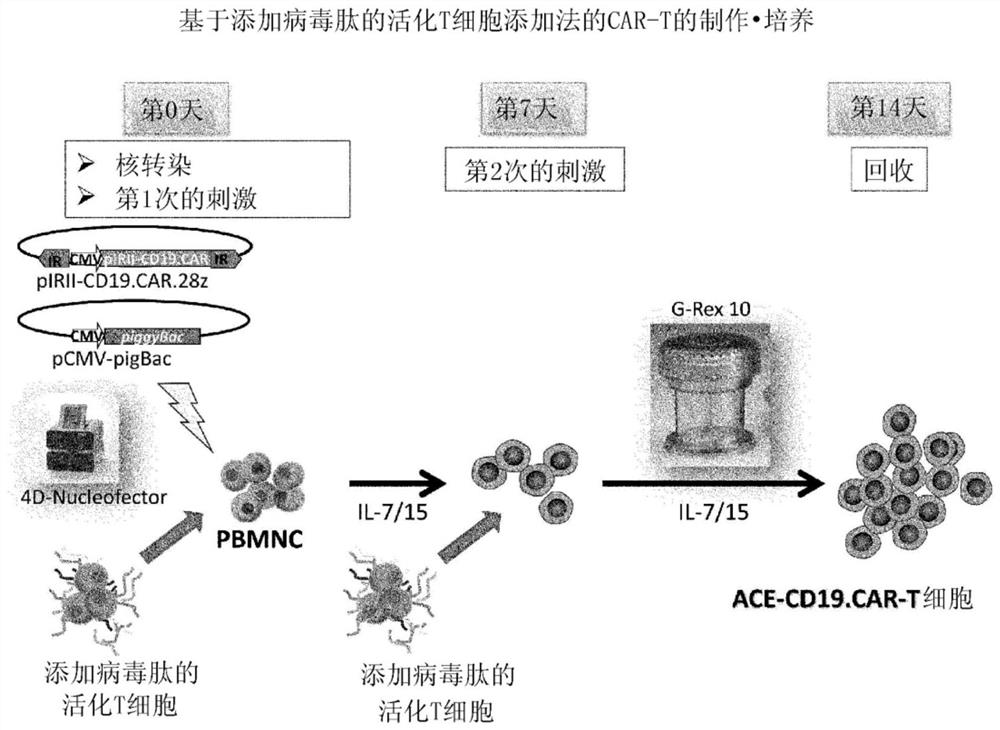
Method for preparing genetically-modified t cells which express chimeric antigen receptor
ActiveCN108138171AExpand clinical applicationGood treatment effectImmunoglobulin superfamilyMammal material medical ingredientsPeptide antigenAntiendomysial antibodies
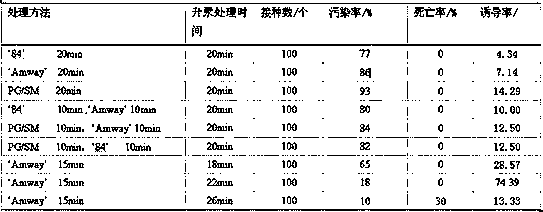
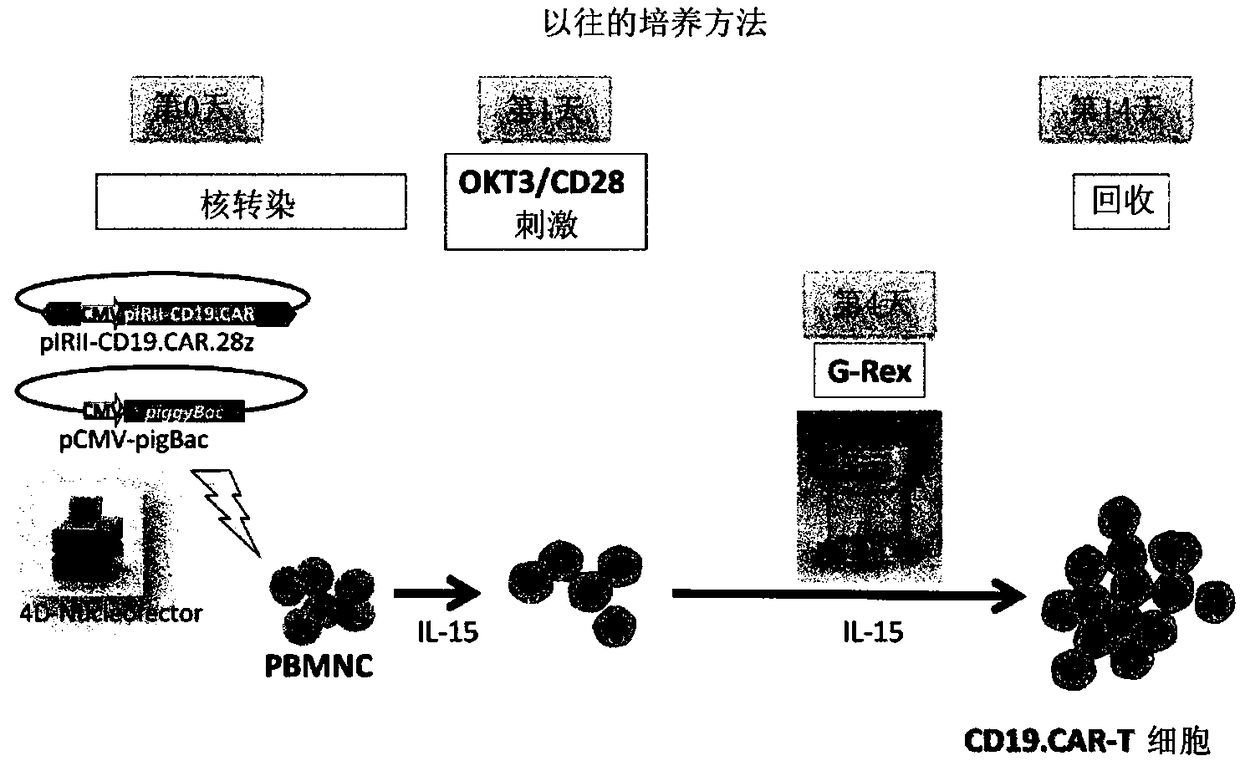
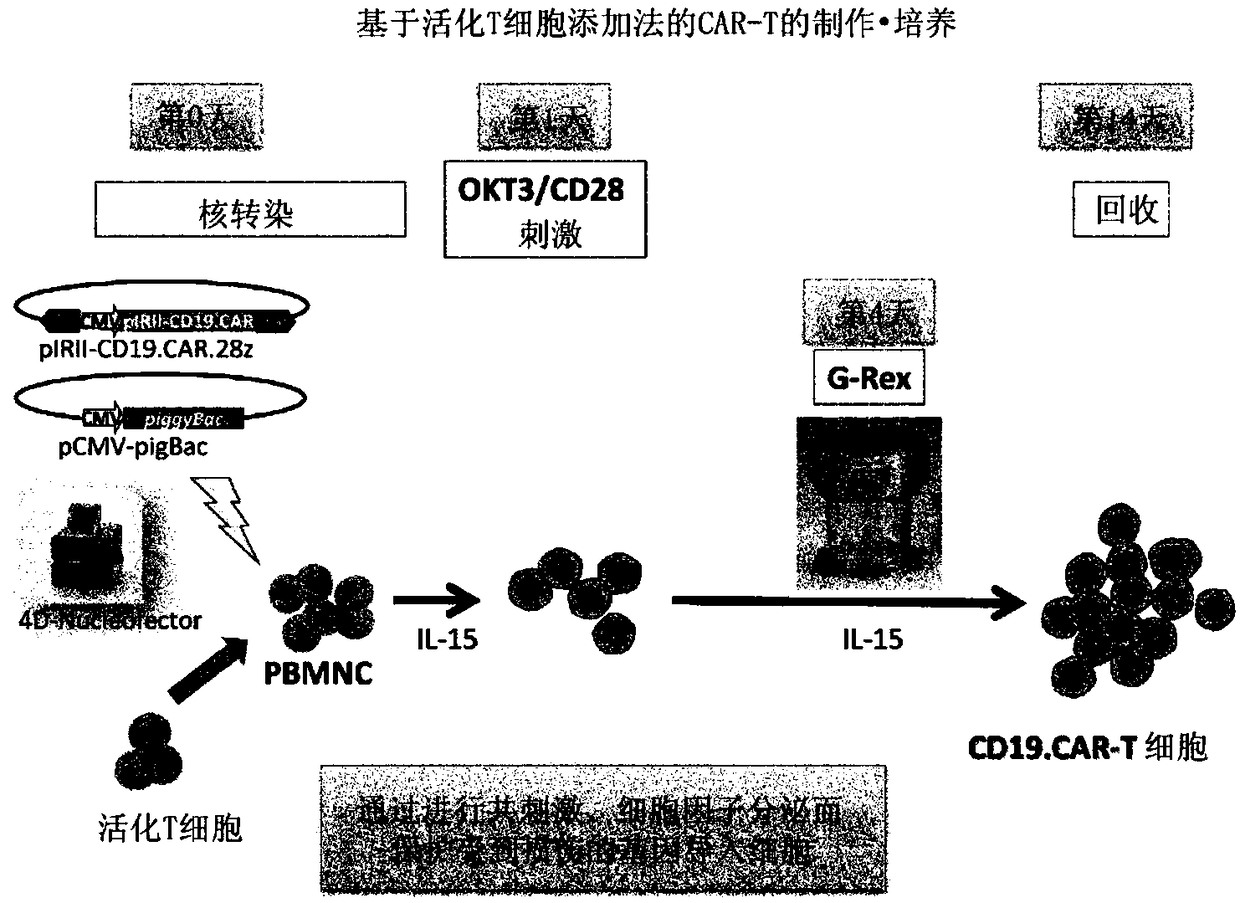
In order to improve the efficiency of gene introduction in CAR therapy employing a transposon technique, provided is a method for preparing genetically-modified T cells which express a chimeric antigen receptor, comprising: (1) a step for preparing non-proliferative cells obtained by stimulating a group of cells including T cells using an anti-CD3 antibody and an anti-CD28 antibody, and thereafter, subjecting the cells to a treatment for causing the cells to lose their proliferation capability; (2) a step for obtaining genetically-modified T cells into which a target antigen-specific chimericantigen receptor gene has been introduced using a transposon technique; (3) mixing the non-proliferative cells prepared at step (1) with the genetically-modified T cells obtained at step (2), and co-culturing the mixed cells while stimulating the mixed cells using the anti-CD3 antibody and the anti-CD28 antibody; and (4) a step for collecting the cultured cells. Also, provided is a method for preparing genetically-modified T cells which express a chimeric antigen receptor, comprising: (i) a step for preparing non-proliferative cells holding a viral peptide antigen, which cells are obtained bystimulating a group of cells including T cells using an anti-CD3 antibody and an anti-CD28 antibody, and thereafter, subjecting the cells to culturing in the presence of the viral peptide antigen anda treatment for causing the cells to lose their proliferation capability; (ii) a step for obtaining genetically-modified T cells into which a target antigen-specific chimeric antigen receptor gene hasbeen introduced using a transposon technique; (iii) mixing the non-proliferative cells prepared at step (i) with the genetically-modified T cells obtained at step (ii), and co-culturing the mixed cells; and (iv) a step for collecting the cultured cells.
Owner:NAT UNIV CORP TOKAI NAT HIGHER EDUCATION & RES SYST
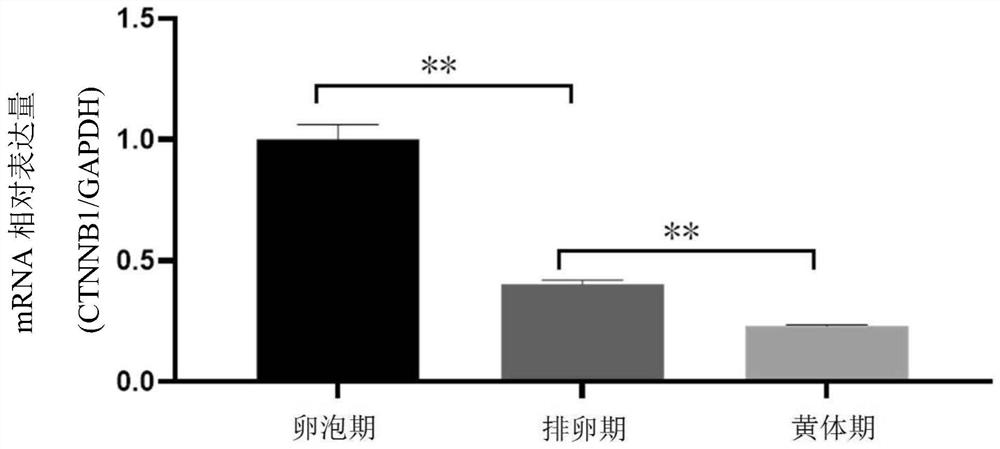
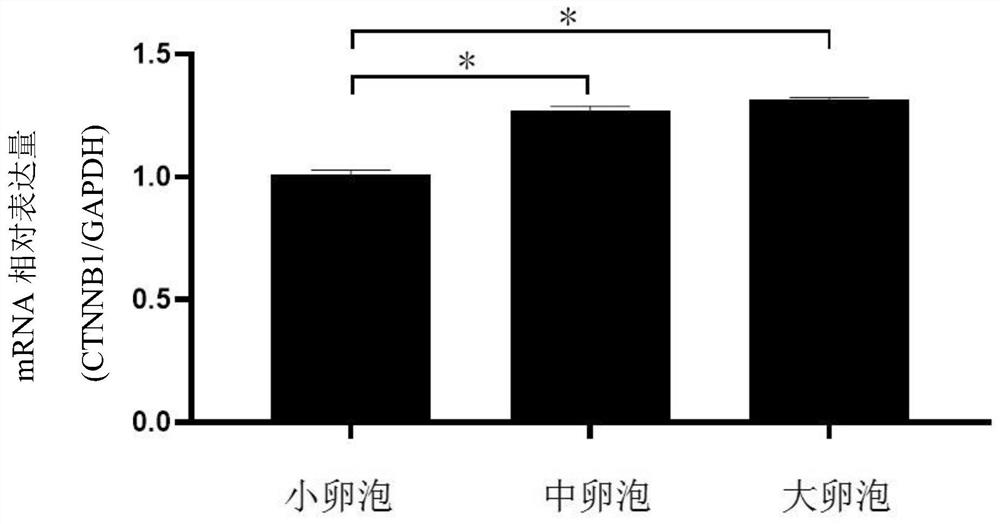
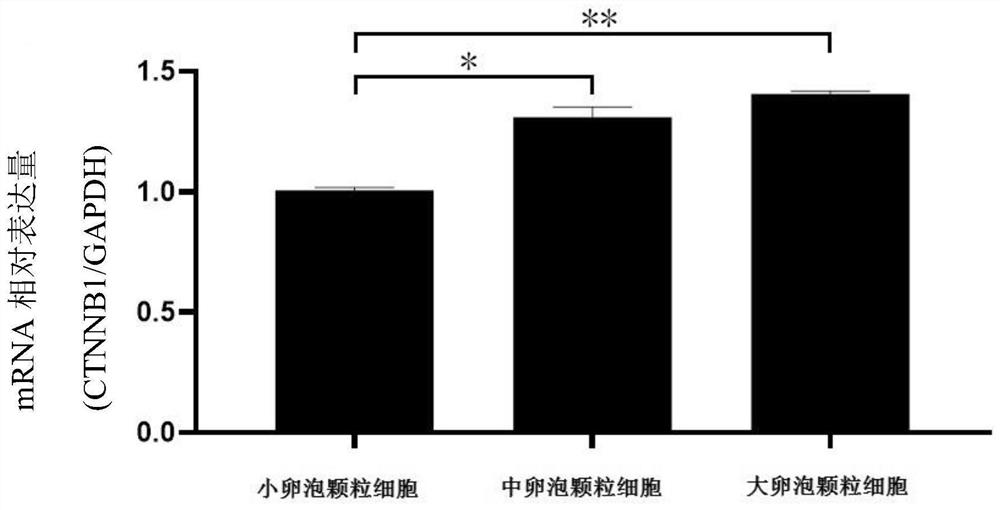
Application of CTNNB1 gene in porcine ovarian granulosa cells
ActiveCN111748559ARelatively high expressionPromote growth and developmentMicrobiological testing/measurementGenetically modified cellsGranular leucocyteOvarian Granulosa Cell
The invention discloses application of a CTNNB1 gene in porcine ovarian granulosa cells, and belongs to the technical field of cell engineering and gene engineering. According to the invention, the relative expression quantity of CTNNB1 mRNA in ovarian tissues, follicles with different sizes and granulosa cells of pigs in different periods, and the proliferation and apoptosis of the granulosa cells after overexpression and inhibition of CTNNB1 and the secretion of steroid hormones are detected. Results show that the CTNNB1 gene participates in promoting the proliferation of granulosa cells andthe secretion of estradiol and inhibiting the apoptosis of granulosa cells and the secretion of testosterone and progesterone, which indicates that the CTNNB1 gene participates in the development andmaturation of ovarian follicles of sows. Materials are accumulated for molecular mechanism research in the sow ovarian follicle development process.
Owner:SOUTH CHINA AGRI UNIV
Fenza No.1 dwarf banana tissue culture rapid propagation method
The invention discloses a Fenza No.1 dwarf banana tissue culture rapid propagation method, and belongs to the field of plant tissue culture. The method comprises the following steps: selecting a plant having green and stout cauloid, the height of 3.2-4.5 m, big fruit clusters, fewer fruit finger conjoined fruits, normal fruit shape and good quality as a mother plant; spraying absorptive buds on the mother plant with a carbendazim and streptomycin mixed liquid, 1 week later, taking the absorptive buds as explants, and inoculating; controlling a differentiation culture temperature to be 27 DEG C-33 DEG C, carrying out differentiation culture for 12-20 days, subculturing, and controlling the total number of subculture generations to be 12-16; and carrying out rooting culture and hardening seedlings. The Fenza No.1 rooting seedlings bred by the technology has more, stout and white root systems, pale yellow green plantlet cauloids, dark green leaves and high provisonal planting survival rate, and grow quickly and neatly; each explant can reproduce about 5000-8000 plantlets within a year, after the plantlets are fix-planted, the seed nature is stable, fewer variant plants appear, and banana farmers have good reflection.
Owner:POMOLOGY RES INST GUANGDONG ACADEMY OF AGRI SCI
Fully human-anti-GPC3 all-molecule IgG antibody and applications thereof
InactiveCN106084041AGood killing effectStrong specificityImmunoglobulins against animals/humansAntibody ingredientsHeavy chainAmino acid
The invention discloses a fully human-anti-GPC3 all-molecule IgG antibody. The amino acid sequence of a light chain variable region is shown by SEQ ID NO.3, or is a conservative variant obtained by conservative mutation of the sequence through adding, deleting, replacing and modifying one or more amino acids; and the amino acid sequence of heavy chain variable region is shown by SEQ ID NO.4, or is a conservative variant obtained by conservative mutation of the sequence through adding, deleting, replacing and modifying one or more amino acids. The invention further discloses applications of the fully human-anti-GPC3 all-molecule IgG antibody. The invention also discloses a gene coded with the fully human-anti-GPC3 all-molecule IgG antibody. The invention further discloses an expression vector comprising the gene and an expression regulation sequence operatively connected with the gene. The invention also discloses a host cell which is transformed by the expression carrier.
Owner:SINOBIOWAY CELL THERAPY CO LTD
Temperature sensitive material preparation method
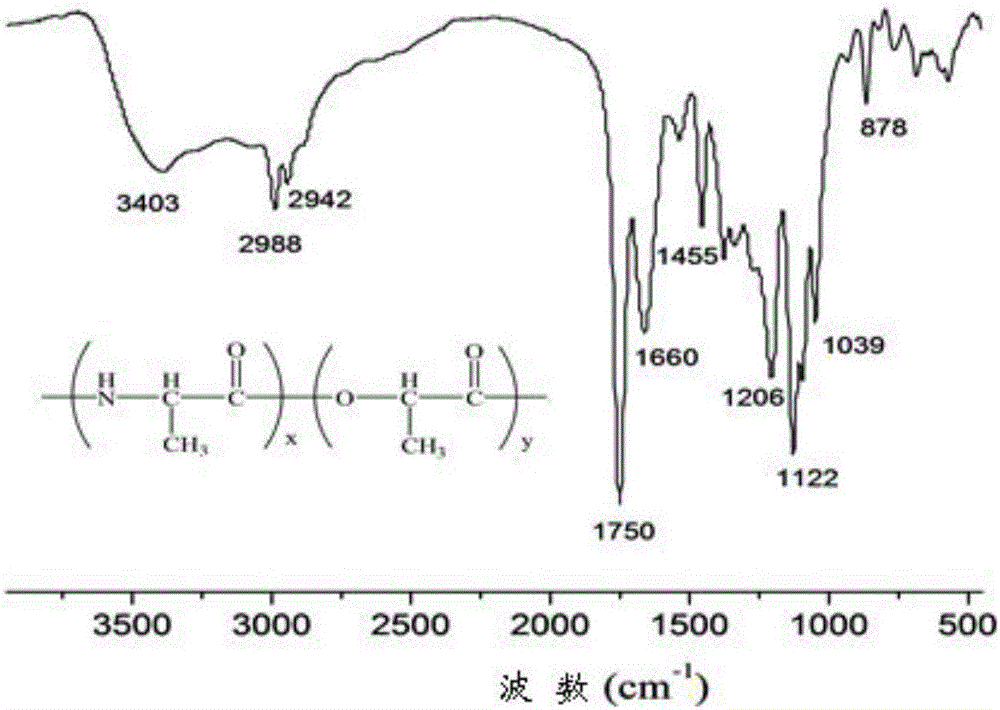
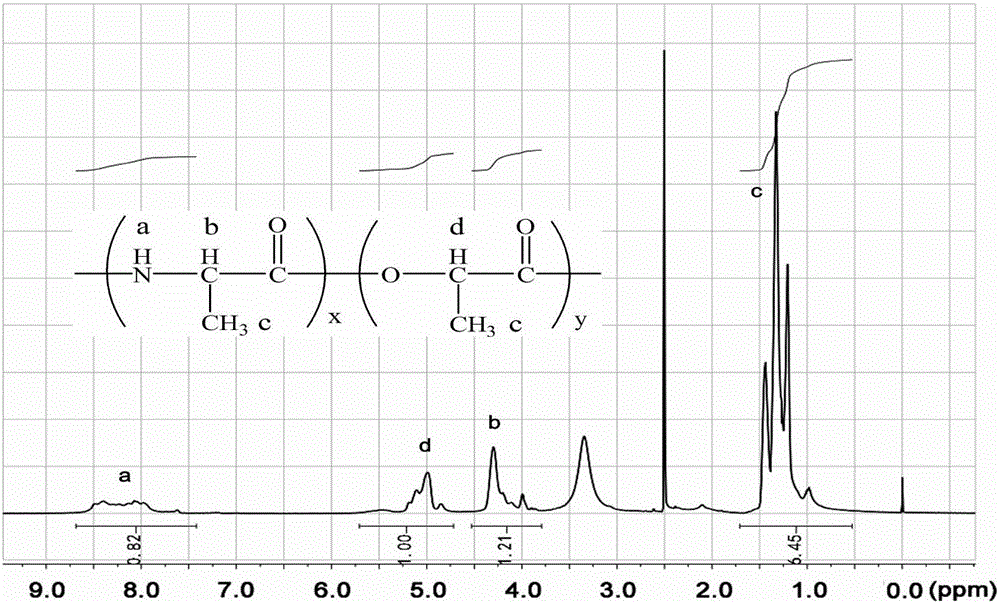
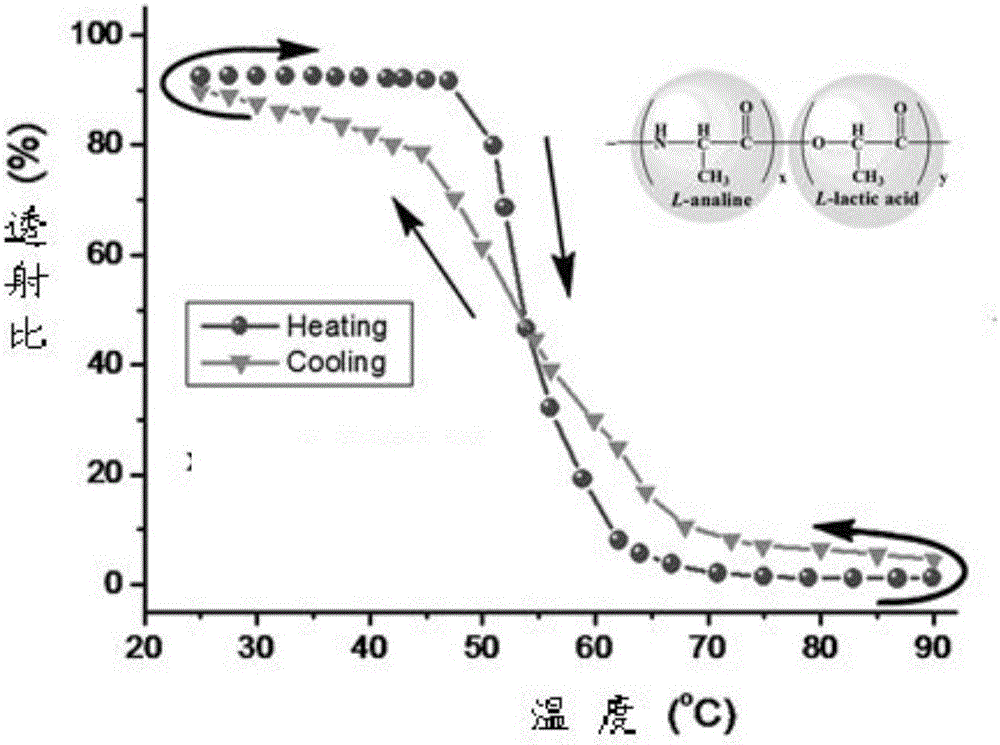
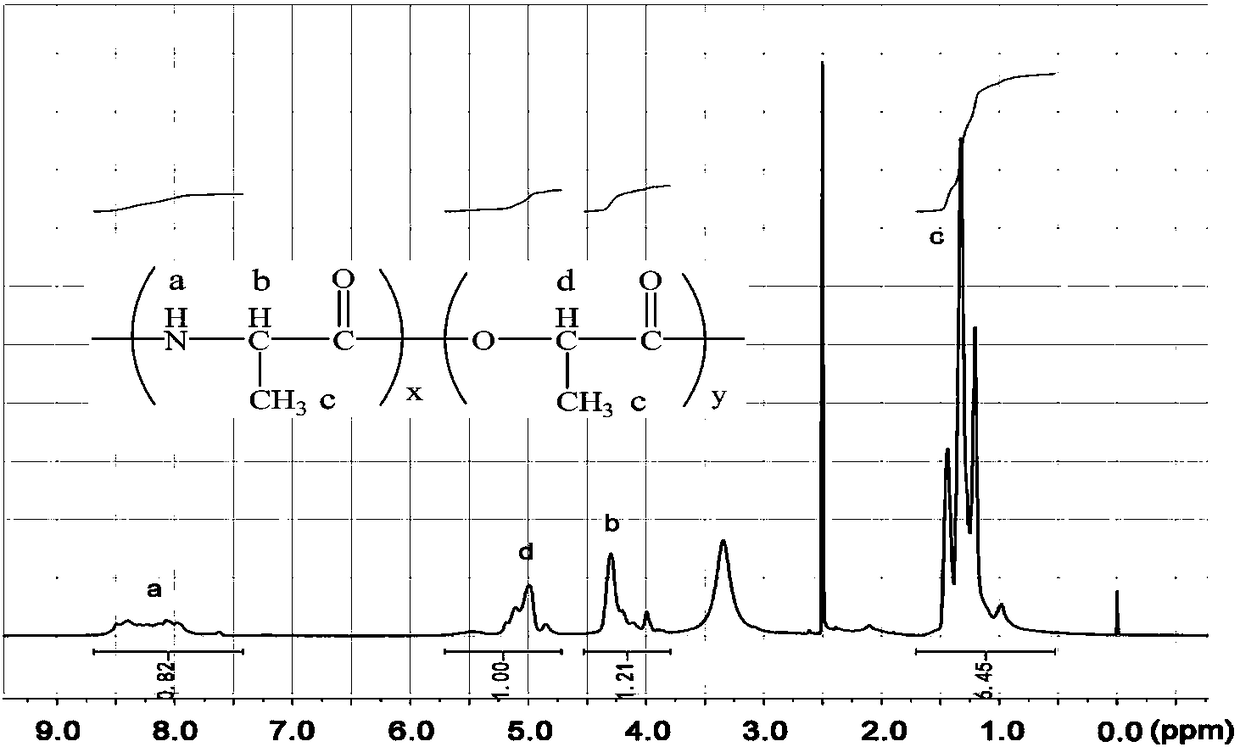
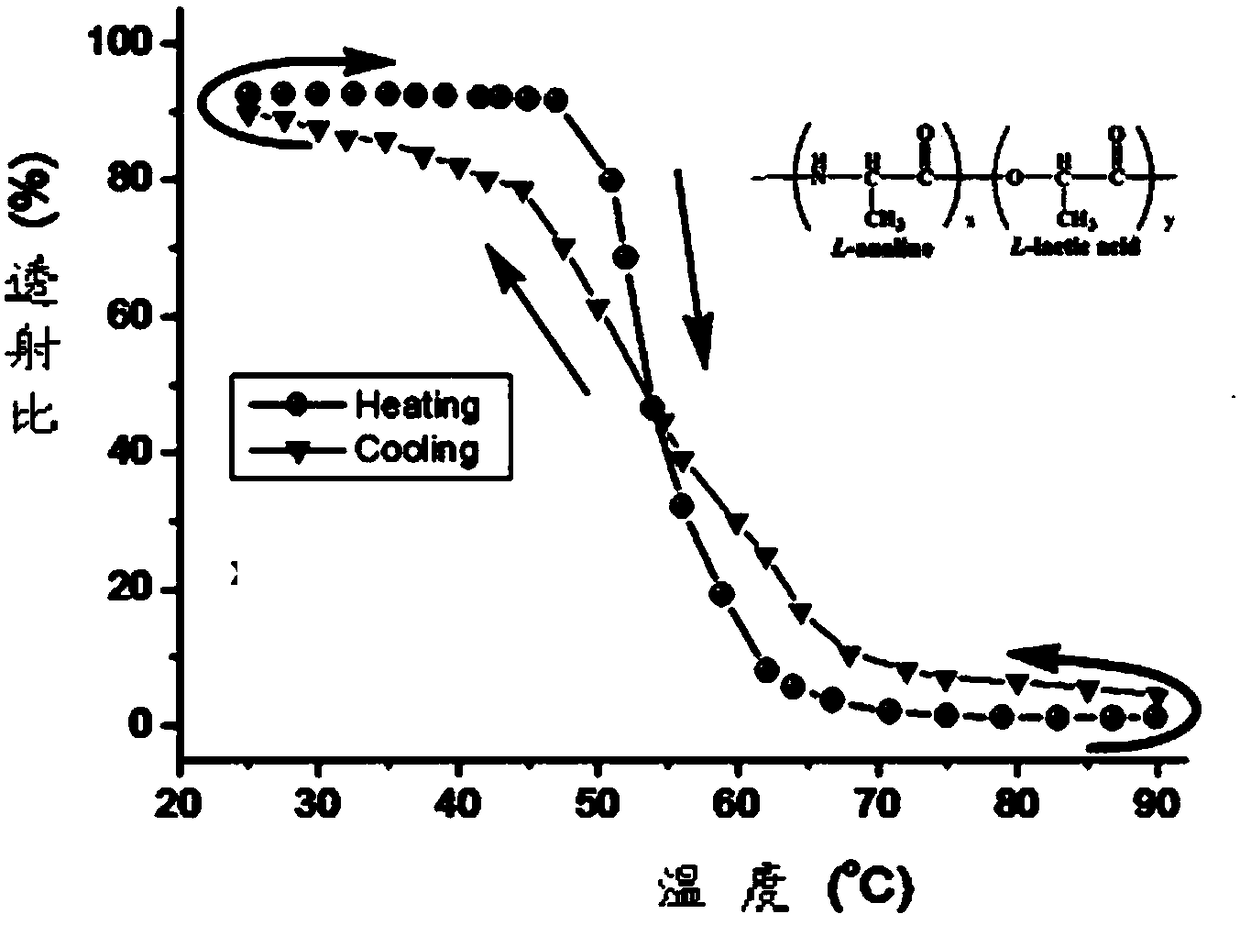
InactiveCN106243349AExcellent reversible responsivenessExcellent temperature sensitive performanceAlanineBiocompatibility Testing
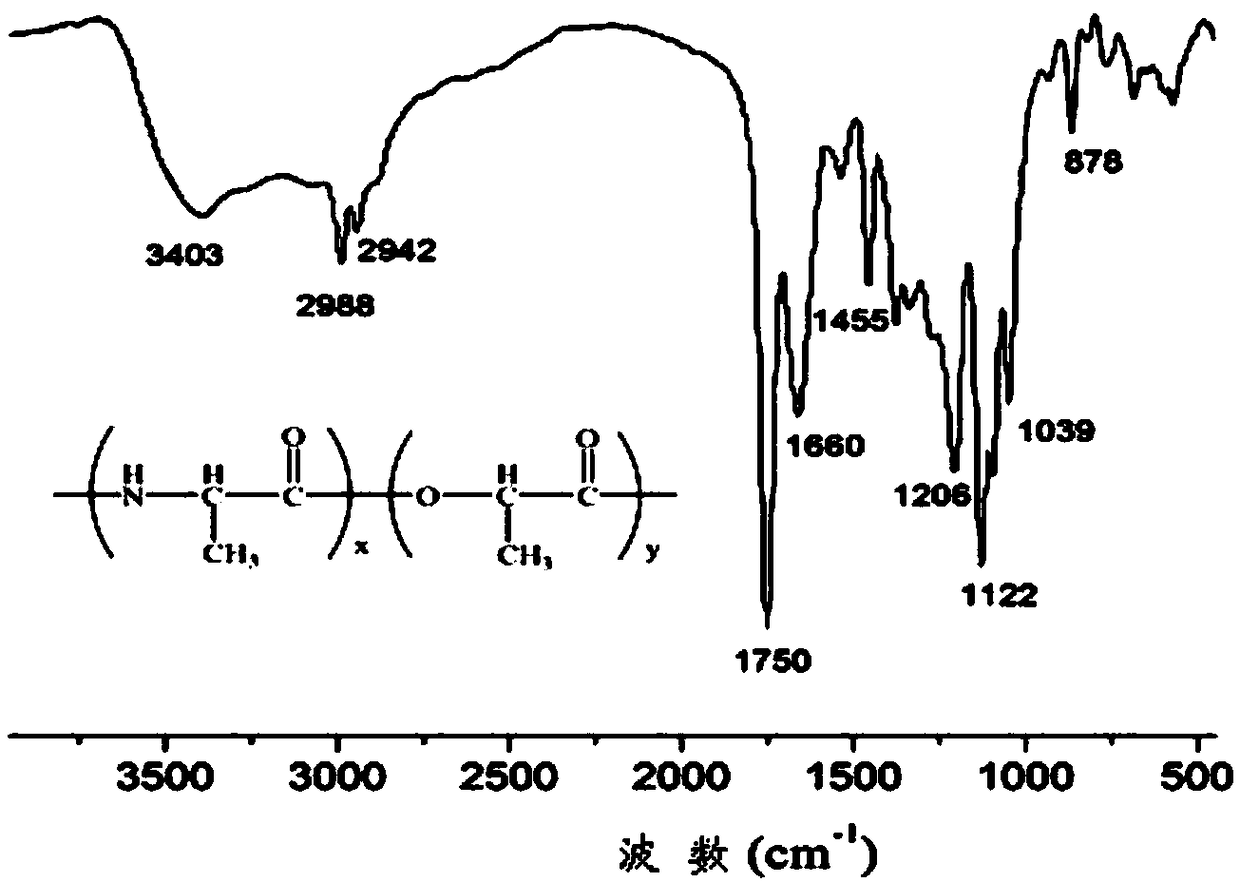
The present invention provides a temperature sensitive material preparation method, which comprises: dehydrating lactic acid to obtain dehydrated lactic acid; and carrying out a copolymerization reaction on the dehydrated lactic acid and alanine under a catalyst condition to obtain the temperature sensitive material. Compared to the existing temperature sensitive polymer material, the temperature sensitive material of the present invention has the following characteristic that the natural bio-friendly alanine and the lactic acid are adopted as the polymerization monomers to directly copolymerize to prepare the polymer material having the temperature sensitive property. The temperature sensitive material of the present invention has excellent temperature sensitivity and biocompatibility.
Owner:HEBEI UNIVERSITY
Application of benzisoselenazole derivative combined with platinum drug to preparation of tumor treatment drugs and postoperative tumor recurrence drugs
PendingCN110856718APrevent proliferationInhibition of proliferation rateHeavy metal active ingredientsAntineoplastic agentsCarboplatinTumor therapy
The invention belongs to the technical field of tumor treatment, and discloses application of a benzisoselenazole derivative combined with a platinum drug to the preparation of tumor treatment drugs and postoperative tumor recurrence drugs. The benziselenazole derivative has a structure as shown in the formula A, the platinum-based anticancer drug is selected from at least one of cisplatin, carboplatin, oxaliplatin, nedaplatin, and the like. The molar ratio of the benziselenazole derivative to the platinum-based anticancer drug is (1-99): (1-99). With the combination of the benzisoselenazole derivative and the platinum drug, the dosage of platinum-based anticancer drug with high toxicity can be effectively reduced and the safety of anticancer drugs can be improved; and Bel-7402 cell apoptosis can be induced by reducing Bcl-2 / Bax protein expression ratio, and the expression of TrxR in tumor tissues can be synergistically inhibited, the proliferation rate of tumor cells after operation can be significantly reduced, and the growth inhibition rate of tumor cells after operation can be improved.
Owner:SHANGHAI YUANXI MEDICINE CORP
Acipenser dabryanus spermatogonium culture solution and application thereof
ActiveCN105316282ASolve the problem that cannot be cultivated in large quantitiesEffective cell platformGerm cellsSpermatogoniumGrowth factor
The invention discloses an acipenser dabryanus spermatogonium culture solution and application thereof. According to the culture solution, a low-serum culture medium which does not have fetal calf serum, adopts various growth factors, nonessential amino acid, sodium pyruvate and other additives as substitutes and is assisted by a small amount of male acipenser dabryanus serum is provided so as to guarantee mass reproduction of spermatogonium and low reproduction rate of a spermatic cell; and the spermatic cell 13d which is cultured to the 13rd day are doped with 25mu M BrdU and is marked as a newly reproduced cell. 24h immunohistochemical experiments indicate that the ratio of germ cell marked cells in newly reproduced cells after BrdU is doped for 24 hours is 85.78 percent. According to the culture solution, the current problem that the acipenser dabryanus spermatogonium passage cannot be massively cultured can be solved, and an effective cell platform is provided to acipenser dabryanus reproductive development researches.
Owner:YANGTZE RIVER FISHERIES RES INST CHINESE ACAD OF FISHERY SCI
Calycosin derivative as well as synthesis method and application thereof
ActiveCN111170980AInhibition of phosphorylation levelsPrevent proliferationOrganic active ingredientsOrganic chemistryHigh concentrationCancer research
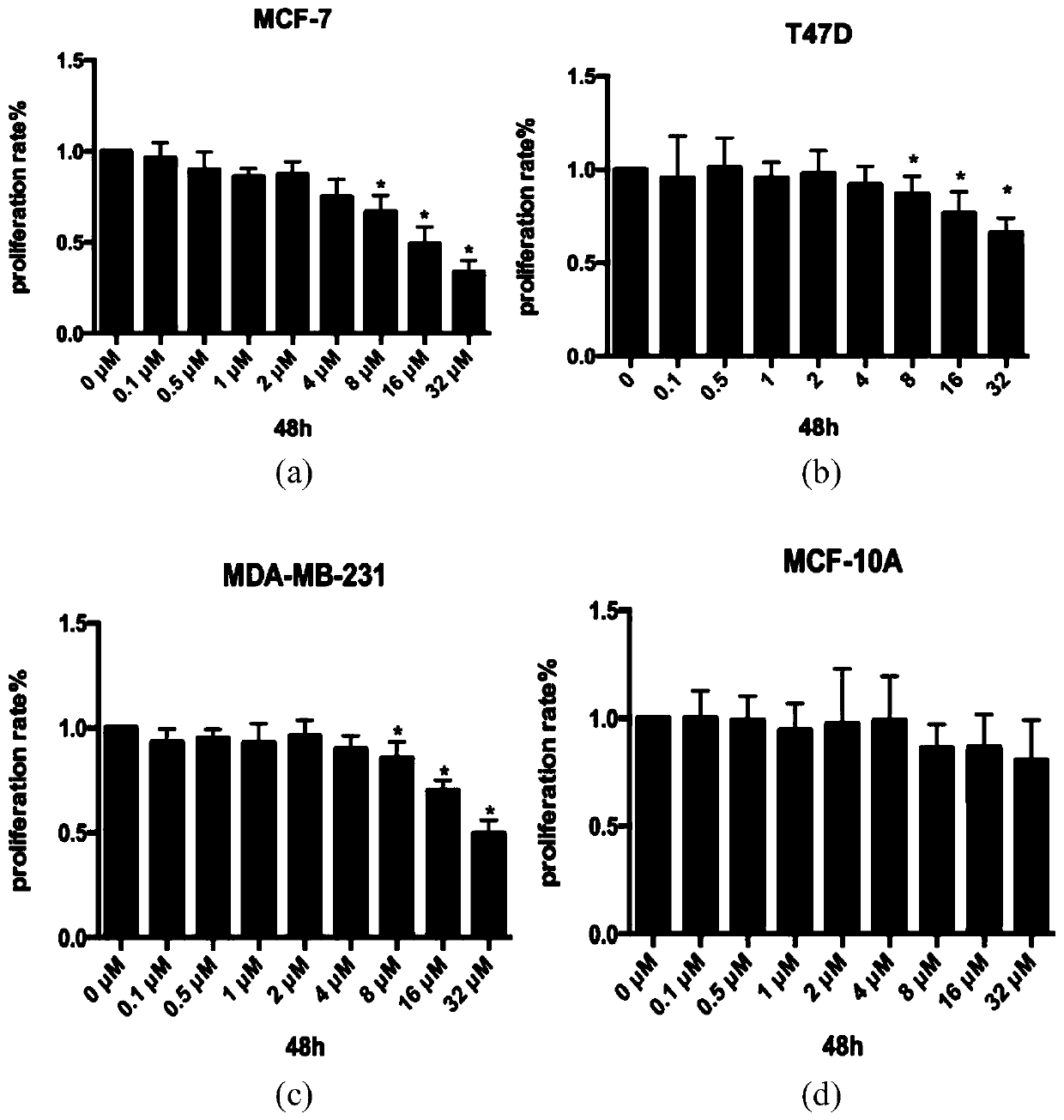
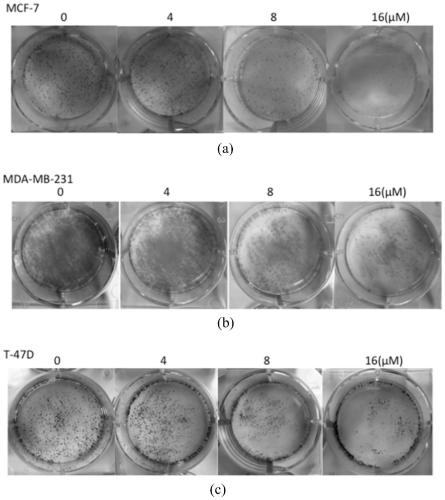
The invention discloses a calycosin derivative as well as a synthesis method and application thereof. The synthesis method of the calycosin derivative mainly comprises the following steps of: dissolving a compound 1 and a compound 2 in an organic solvent, adding an acid-binding agent, and reacting under a heating condition to obtain a target crude product. Experimental results of the applicant show that the calycosin derivative can inhibit proliferation of ER positive breast cancer cells and ER negative breast cancer cells at the same time; along with the increase of the concentration of the derivative, the proliferation rate of breast cancer cells is gradually reduced, the inhibition effect is the most obvious at high concentration, and no influence is caused to normal breast cells MCF-10A.
Owner:GUILIN MEDICAL UNIVERSITY
Method for establishing Caihong populus regeneration system by taking petioles as explants
InactiveCN112335550AReduce reproductionShorten speedHorticulture methodsPlant tissue culturePlantletSeedling
The invention provides a method for obtaining regenerated plants by inducing calluses by using petioles of Caihong populus as explants. The method comprises the following steps of firstly, selecting explants, disinfecting with 0.1% HgCl2 for 12 minutes, inoculating into an induction culture medium, carrying out callus induction, controlling the culture temperature at 24-26 DEG C, culturing at theillumination time of 10-14h / day and the illumination intensity of 1400-1800lx, transferring obtained Caihong populus calluses into a differential medium to be cultured for 30 days, and finally, inoculating aseptic seedlings of the Caihong populus into a rooting medium to be subjected to rooting culture for 25 days. The method can effectively solve the problem of in-vitro rapid propagation of the Caihong populus.
Owner:INST OF BOTANY JIANGSU PROVINCE & CHINESE ACADEMY OF SCI
Method for establishing Caihong poplar regeneration system by taking leaves as explants
ActiveCN112369331AReduce reproductionGet efficientlyHorticulture methodsPlant tissue cultureSeedlingCulture mediums
The invention belongs to the field of tissue culture propagation, and discloses a method for establishing a Caihong poplar regeneration system by taking leaves as explants. The method comprises the following steps that firstly, the explants are selected, then are disinfected with 0.1% of HgCl2 for 12 minutes and are inoculated into an induction culture medium for adventitious bud induction, the culture temperature is controlled within 24-26 DEG C, then culture is carried out for 25 days under the conditions that the illumination time is 10-14 h / d and the illumination intensity is 1400-1800 lx,then seedlings of the adventitious buds of the Caihong poplar are cut and transferred into a subculture medium for culture, and finally aseptic seedlings of the Caihong poplar are inoculated into a rooting culture medium for rooting culture for 25 days. According to the method, the problem of in-vitro rapid propagation of the Caihong poplar can be effectively solved.
Owner:INST OF BOTANY JIANGSU PROVINCE & CHINESE ACADEMY OF SCI
EGCG-CoPt NPs-Apt nanoparticles as well as preparation method and application thereof
PendingCN112933241AInhibit migrationInhibit transferOrganic active ingredientsPowder deliveryAptamerOncology
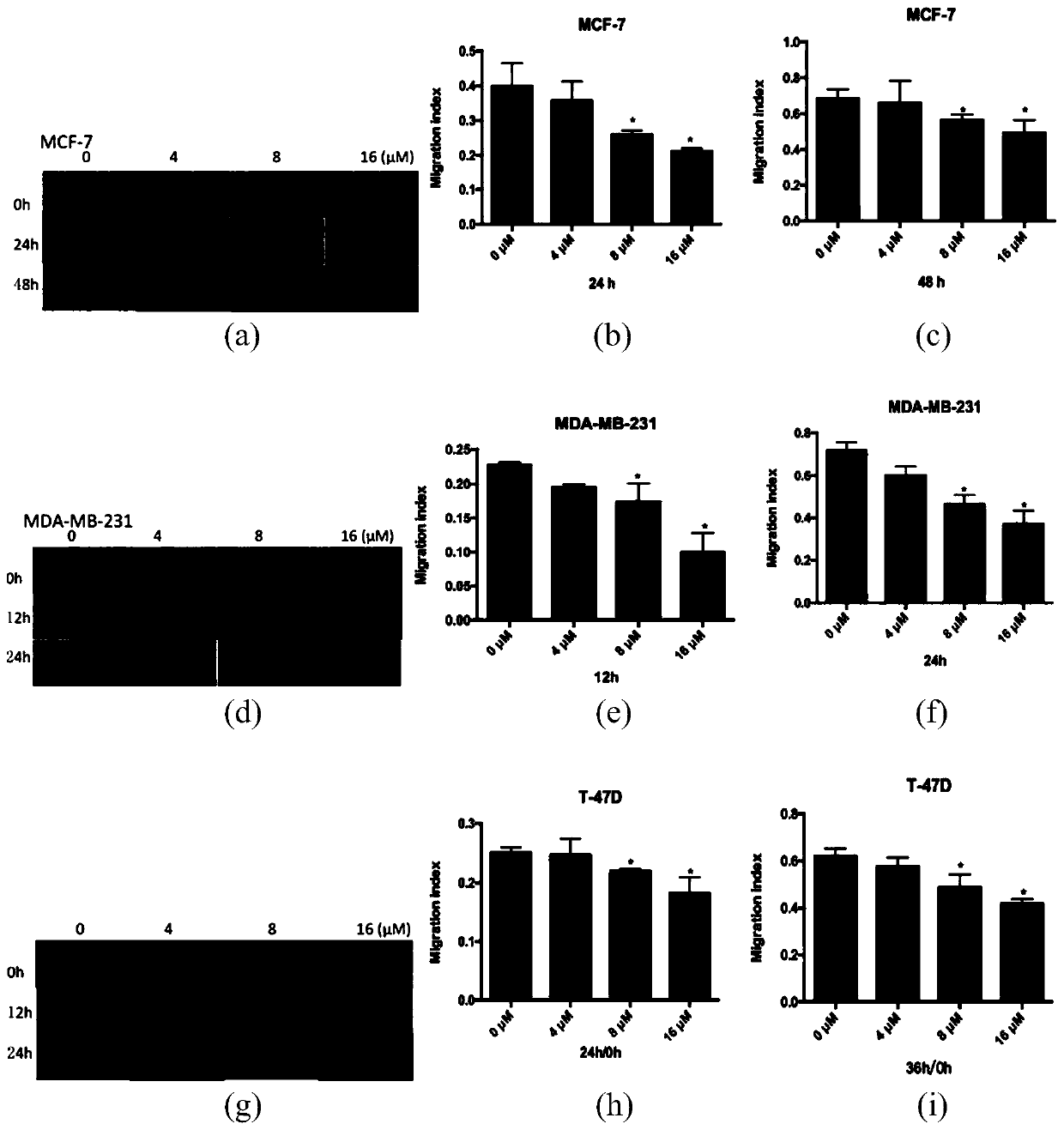
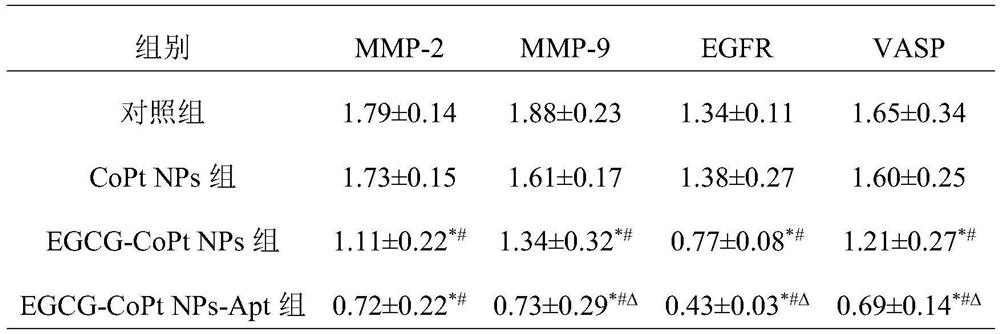
The invention belongs to the field of biological medicine, and relates to an EGCG-CoPtNPs-Apt nanoparticle as well as a preparation method and application thereof. According to the EGCG-CoPt NPs-Apt nanoparticles as well as the preparation method and application thereof, EGCG is taken as a template, EGCG-CoPtNPs nanoparticles are synthesized in a green manner, and then the surfaces of the EGCG-CoPtNPs nanoparticles are modified with specific nucleic acid aptamers AS1411 of triple negative breast cancer cells, so that the composite EGCG-CoPtNPs-Apt nanoparticles with a molecular recognition function are formed. According to the EGCG-CoPtNPs-Apt nanoparticles prepared by the invention, the dual targeting effect of the nanoparticles and the nucleic acid aptamers, the proliferation rate and the transmembrane effect of the triple negative breast cancer cells MDA-MB-231 can be remarkably reduced, meanwhile, the expression quantity of tumor cell transfer factors MMP-2, MMP-9, EGFR and VASP is reduced, tumor cell metastasis is inhibited, and a foundation is laid for clinical triple-negative breast cancer diagnosis and treatment integration.
Owner:福州市第二医院
Method for carrying out tissue culture and rapid propagation by using lycoris sprengeri comes ex baker plateau as explant
InactiveCN109329060AReduce reproductionEfficient reproductionHorticulture methodsPlant tissue cultureBudPlateau
The invention provides a method for inducing adventitious buds to obtain a regeneration plant by using lycoris sprengeri comes ex baker plateau as an explant. The method comprises the following steps:selection and disinfection of the explant, induction of the adventitious buds, subculture and proliferation, induction of roots and transplanting of tissue culture seedlings. The method disclosed bythe invention has the characteristics that the induced germination rate reaches 98 percent, the initial generation of single plant proliferative adventitious buds reaches 15 times or above and the rooting rate reaches 99 percent. The problem of in vitro rapid propagation of lycoris sprengeri comes ex baker can be effectively solved through the method.
Owner:INST OF BOTANY JIANGSU PROVINCE & CHINESE ACADEMY OF SCI
Nutritional repair composition for enhancing effect after medical beautifying treatment
InactiveCN111481458AImprove whiteningReduce saggingCosmetic preparationsToilet preparationsBiotechnologyNutrition
The invention discloses a nutritional repair composition for strengthening the effect after medical beautifying treatment. The invention belongs to the technical field of biological medicine, the composition is prepared from the following substances in parts by weight: 40 to 50 parts of elastin extract, 5 to 8 parts of fish maw, 2 to 5 parts of chitosan, 0.5 to 1 part of green tea extract, 6 to 9parts of fruit seed extract, 0.2 to 0.4 part of composite vitamin, 0.1 to 0.3 part of zinc gluconate, 0.2 to 0.5 part of citric acid, 0.1 to 0.2 part of L-malic acid and 50 to 55 parts of auxiliary materials. The nutritional repair composition can enhance the recovery speed and recovery capability of the scar skin of a patient, strengthens the effect recovery after medical and art treatment, has agood comprehensive use effect, and is green and safe.
Owner:上海清冬闻晓电子商务有限公司
Preservative method of haematococcus pluvialis pulp or mashed haematococcus pluvialis
InactiveCN107535799AWon't rotReduce breeding rateClimate change adaptationFood preservationPreservativeMicroscopic exam
The invention discloses a preservative method of haematococcus pluvialis pulp or mashed haematococcus pluvialis. The method specifically comprises the following steps that firstly, the pH value of thehaematococcus pluvialis pulp of which the concentration is 3g / L-10g / L is regulated with an acid and an alkali to be smaller than or equal to 6, then 0.05%-0.5% of a frequently-used preservative is added to the haematococcus pluvialis pulp, uniform mixing is performed, after 3 days, any colonies are not found to be formed, the haematococcus pluvialis pulp is not rotten or generates bad smell, andthrough microscopic examination, haematococcus pluvialis cells are found to be complete without rupture. According to the preservative method disclosed by the invention, the condition that haematococcus pluvialis liquid is rotten or generates bad smell in the process of concentration or before spray drying can be effectively avoided, the condition that the haematococcus pluvialis cells are damagedand ruptured is also avoided, the product quality cannot be influenced, and the preservative method is low in cost and simple in operation flows.
Owner:山东鸣惠生物科技股份有限公司
A method for establishing the regeneration system of long-tube Lycoris
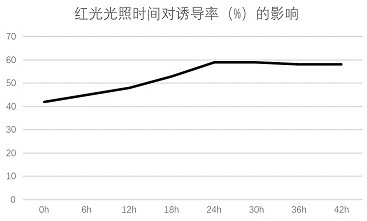
ActiveCN112385547BInduction has no substantial effectImprove the induction effectPlant tissue cultureHorticulture methodsLycoris radiataAnthesis
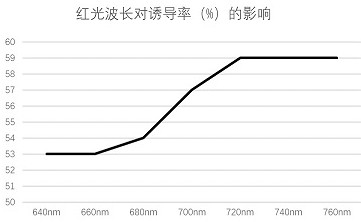
The invention provides a method for establishing a regeneration system of Lycoris long tube, which comprises the following steps: firstly, taking the apex of the flower axis of Lycoris long tube at the initial flowering stage as an explant to induce adventitious buds, controlling the culture temperature at 24-26°C, in the dark Medium culture for 24 hours, then red light culture for 1 day + normal culture for 44 days, then cut the obtained long-tube Lycoris adventitious shoots and put them into the subculture medium for 25 days, and finally put the long-tube Lycoris test-tube seedlings into rooting culture Rooting culture was carried out in the base for 15 days. The method can effectively solve the problem of rapid propagation of Lycoris longifolia in vitro.
Owner:INST OF BOTANY JIANGSU PROVINCE & CHINESE ACADEMY OF SCI
A kind of preparation method of thermosensitive material
InactiveCN106243349BExcellent reversible responsivenessExcellent temperature sensitive performanceMaterials preparationBiocompatibility Testing
The present invention provides a temperature sensitive material preparation method, which comprises: dehydrating lactic acid to obtain dehydrated lactic acid; and carrying out a copolymerization reaction on the dehydrated lactic acid and alanine under a catalyst condition to obtain the temperature sensitive material. Compared to the existing temperature sensitive polymer material, the temperature sensitive material of the present invention has the following characteristic that the natural bio-friendly alanine and the lactic acid are adopted as the polymerization monomers to directly copolymerize to prepare the polymer material having the temperature sensitive property. The temperature sensitive material of the present invention has excellent temperature sensitivity and biocompatibility.
Owner:HEBEI UNIVERSITY
Method for establishing lycoris longituba regeneration system through callus approach
ActiveCN112385547AReduce reproductionReduce proliferation rateHorticulture methodsPlant tissue cultureLycoris radiataSeedling
The invention provides a method for establishing a lycoris longituba regeneration system through a callus approach. The method comprises the following steps of taking healthy leaves of aseptic seedlings of lycoris longituba to be inoculated into an induction culture medium for callus induction, controlling the culture temperature at 24-26 DEG C, and culturing for 30 days under the conditions thatthe illumination time is 10-14 h / day and the illumination intensity is 1400-1800lx; then transferring obtained controlling calluses into a differential medium to be cultured for 30 days; and finally,inoculating aseptic seedlings of colored leaf poplar into a rooting medium to be subjected to rooting culture for 20 days. By means of the method, the problem of in-vitro rapid propagation of the lycoris longituba can be effectively solved.
Owner:INST OF BOTANY JIANGSU PROVINCE & CHINESE ACADEMY OF SCI
Chinese medicinal composition for dispelling scars and preparation method thereof
ActiveCN102091258BPrevent contractureReduce deformityAnthropod material medical ingredientsDermatological disorderScar itchingOral medication
The invention discloses a Chinese medicinal composition for dispelling scars. The composition is prepared from oral administration medicaments and externally-applied medicaments, wherein the oral administration medicaments comprise but are not limited to the following raw material medicaments: angelica dahurica, peach seed, largehead atractylodes rhizome, job stears, Chinese angelica, Szechuan lovage rhizome, dipsacus asperoides, glabrous sarcandra herb, achyranthes root, earthworm, ground beetle, codonopsis pilosula, rhizoma drynariae, williams elder twig and white paeony root; and the externally-applied medicaments comprise but are not limited to the following raw material medicaments: dragon's blood, myrrh, safflower, Chinese angelica, suberect spatholobus stem, rose mallow root, pseudoginseng root, dipsacus asperoides, spreading hedyotis herb, frank incense, angelica dahurica, pearl, root of red-rooted salvia, williams elder twig, loofah sponge, rose and cortex moutan. The Chinesemedicinal composition is used for treating various burn scares and various scars formed after surgery and trauma and has the effects of activating blood and dissolving stasis, relaxing tendons and activating collaterals, detoxicating and diminishing swelling, softening hardness to dissipate stagnation, inhibiting scar hyperplasia, relieving scar itching, and softening the scars.
Owner:江西博屾医疗器械有限公司
A block anaerobic treatment method for organic wastewater
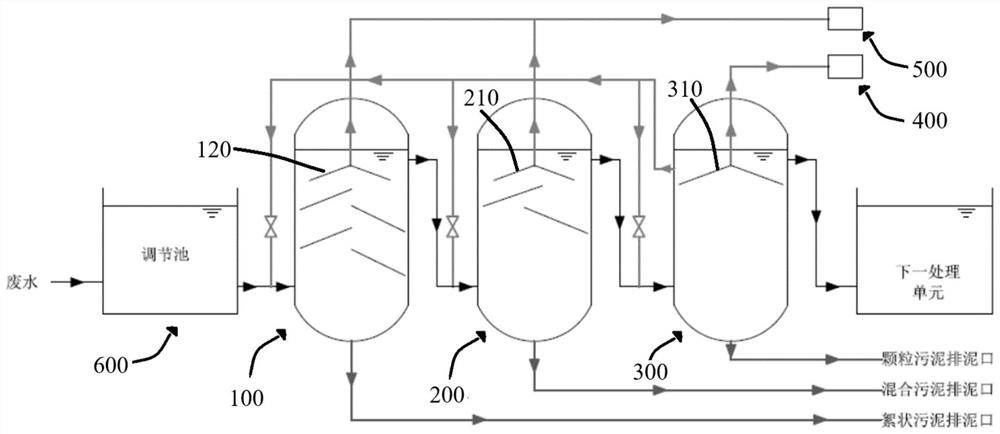
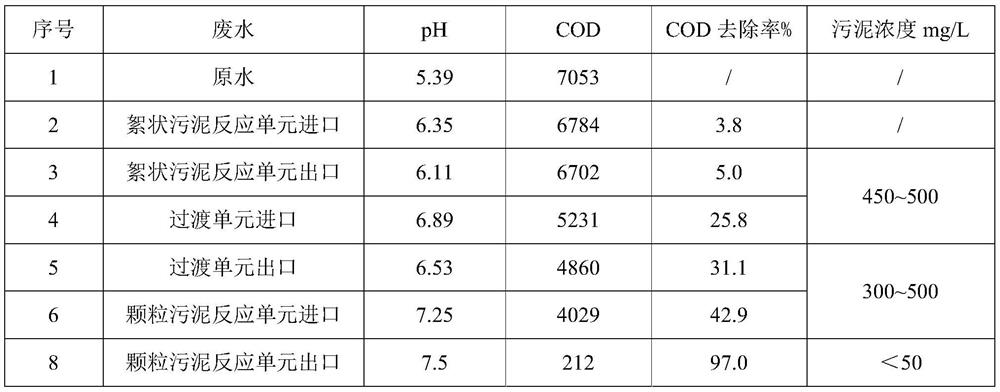
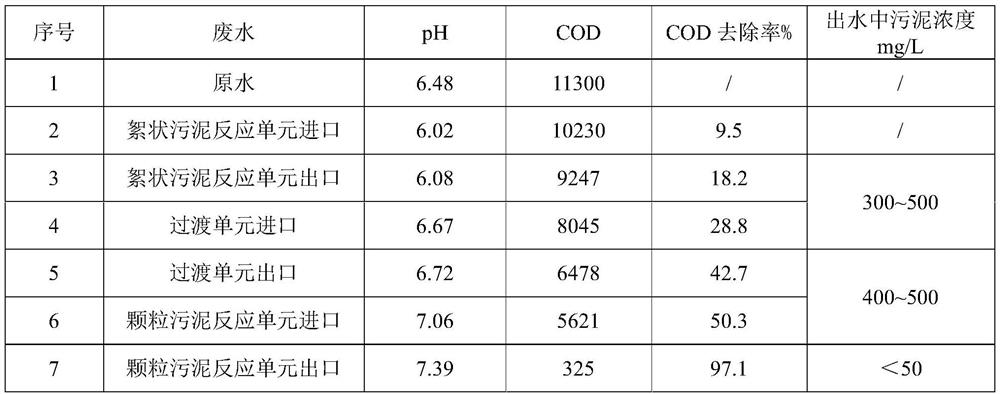
ActiveCN112850886BImprove methane production efficiencyImprove reaction efficiencyWater treatment parameter controlBiological treatment regulationTreatment unitWater treatment
The invention discloses a block-based anaerobic treatment method for organic wastewater, which belongs to the technical field of water treatment. It includes the following steps: 1) passing the water body to be treated into a flocculent sludge treatment unit inoculated with flocculent sludge for treatment; 2) passing 1) effluent into a transition unit for treatment, and the transition unit contains flocculent Sludge and granular sludge; 3) passing 2) the effluent into a granular sludge treatment unit inoculated with granular sludge for treatment; wherein part of the effluent of the granular sludge treatment unit is returned to the flocculent sludge treatment unit and / or Or transition unit and / or granular sludge treatment unit. According to the advantages of different sludges, the present invention sets different sludge partitions, precisely controls the process parameters at different stages, greatly improves the treatment efficiency of the anaerobic process, and increases the output of granular sludge.
Owner:NANJING UNIV +1
Preparation method of genetically modified T cells expressing chimeric antigen receptors
ActiveCN108138171BExpand clinical applicationGood treatment effectImmunoglobulin superfamilyMammal material medical ingredientsPeptide antigenAntiendomysial antibodies
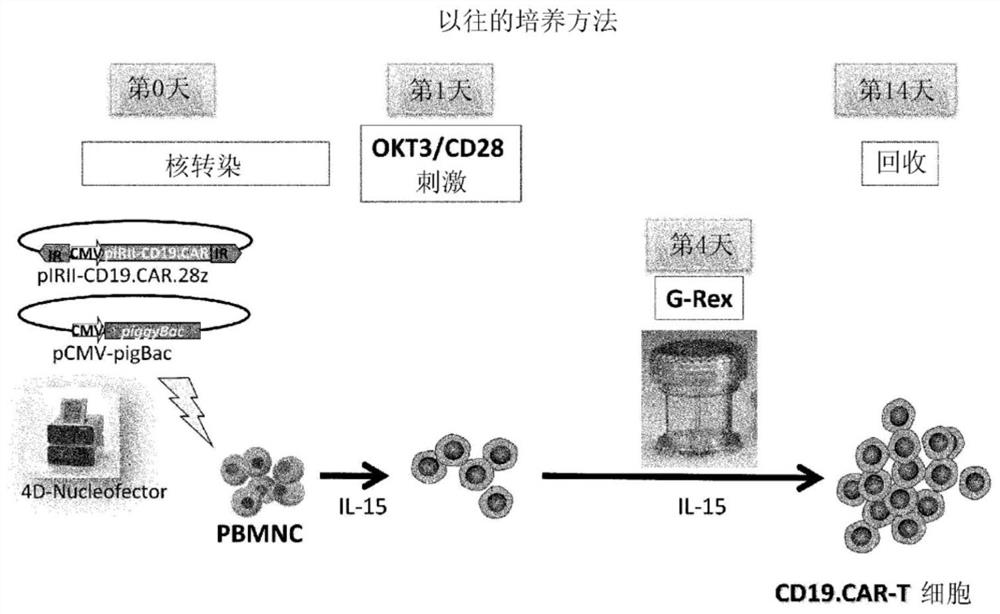
The present invention realizes the improvement of the gene introduction efficiency of CAR therapy using the transposon method. The present invention provides a method for preparing genetically modified T cells expressing chimeric antigen receptors, comprising the following steps: (1) preparing non-proliferative cells, wherein the non-proliferative cells are obtained by using anti-CD3 antibodies and It is obtained by stimulating the cell population containing T cells with an anti-CD28 antibody, and then treating the proliferation ability; (2) obtaining the genetically modified T cells with the target antigen-specific chimeric antigen receptor gene introduced by the transposon method The step of cells; (3) mixing the non-proliferative cells prepared in step (1) and the genetically modified T cells obtained in step (2), and co-cultivating them while stimulating with anti-CD3 antibody and anti-CD28 antibody step; (4) the step of recovering the cultured cells. In addition, the present invention provides a method for preparing a genetically modified T cell expressing a chimeric antigen receptor, comprising the following steps: (i) preparing a non-proliferative cell retaining a viral peptide antigen, said viral peptide retaining Antigen non-proliferative cells are obtained by culturing in the presence of viral peptide antigens and incapacitating the proliferative ability after stimulating a T-cell-containing cell population with an anti-CD3 antibody and an anti-CD28 antibody; (ii) A step of obtaining a genetically modified T cell introduced with a target antigen-specific chimeric antigen receptor gene by a transposon method; (iii) combining the non-proliferative cells prepared in step (i) and the gene obtained in step (ii) The step of modifying T cell mixing and co-cultivation; (iv) the step of recovering the cultured cells.
Owner:NAT UNIV CORP TOKAI NAT HIGHER EDUCATION & RES SYST
Anti-viral pneumonia medicine and preparation method thereof
InactiveCN112546177AMedication is simpleIntuitive effectDispersion deliveryAntiviralsTracheitisDisease
An anti-viral pneumonia medicine relates a medicament for treating viral pneumonia. The medicine consists of folium mori, folium perillae, herba artemisiae, radix puerariae, dried rhizoma phragmitis,herba houttuyniae, fructus forsythiae, flos lonicerae, semen armeniacae amarum, radix peucedani, radix platycodi, bulbus fritillariae thunbergii, fructus arctii, radix scrophulariae, radix adenophorae, massa medicata fermentata, roasted rhizoma atractylodis macrocephalae, herba agastaches, rhizoma osmundae, fructus amomi rotundus, poria and radix glycyrrhizae. The anti-viral pneumonia medicine issimple to use, has a rapid effect, basically takes effect by one dose, has excellent effects of treating lung diseases including viral pneumonia, cough, expectoration, rhinitis, laryngitis, trachitis,bronchitis, pneumonia and the like and preventing and treating lung cancer, and does not have any toxic or side effects. According to the anti-viral pneumonia medicine, the ancient processing technology and the like are adopted to perform high-purity extraction on biological components beneficial to resisting viruses, relieving cough and asthma, clearing heat, eliminating phlegm, diminishing inflammation, easing pain, choleresis increasing, protecting liver, improving immunity and resisting cancers, and thus, the biological components are easily absorbed by the human body.
Owner:王丽敏
Application of ginsenoside to wound repair
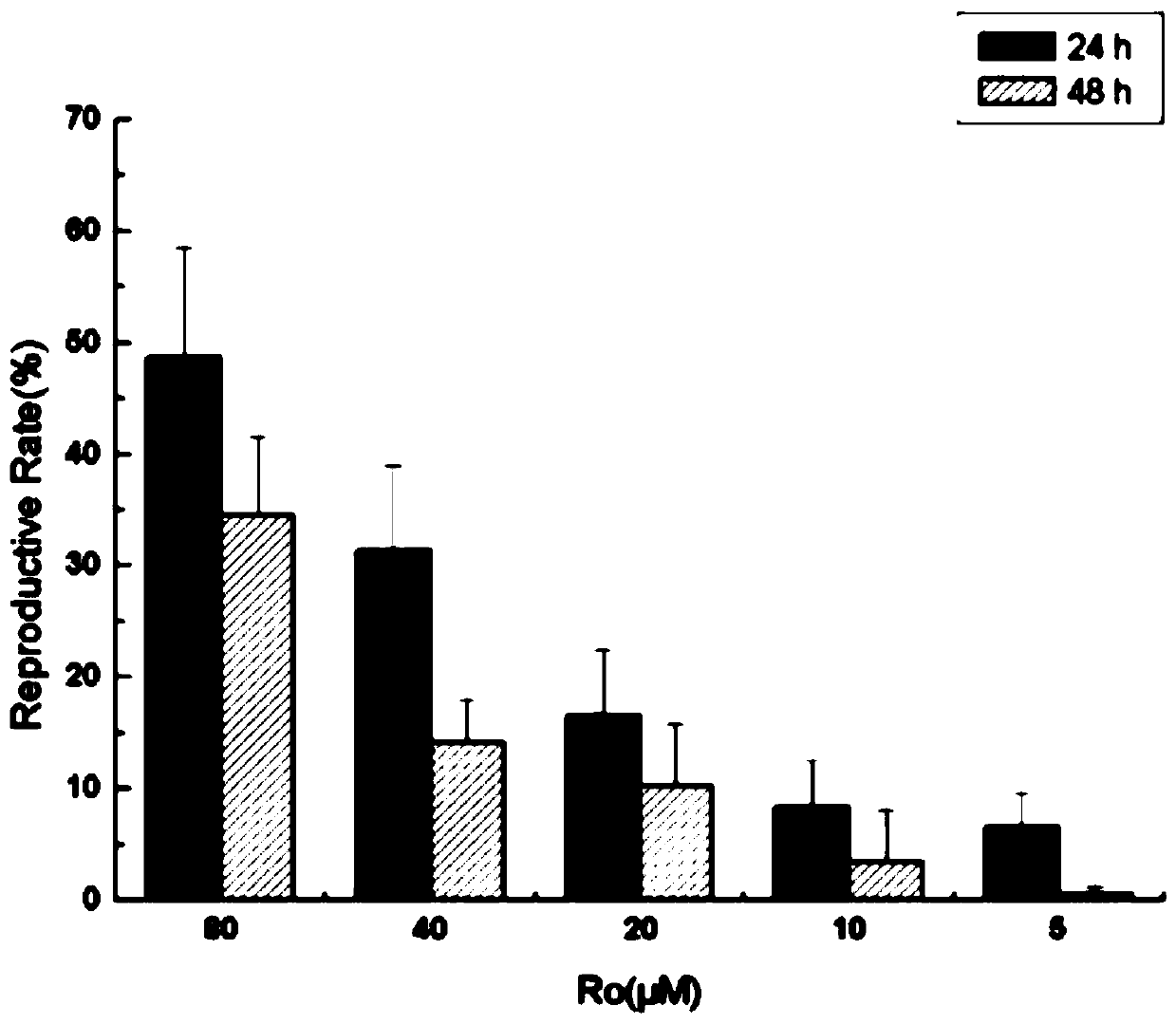
PendingCN111450106APromotes significant proliferationReduce proliferation rateOrganic active ingredientsDermatological disorderPharmaceutical drugPharmacology
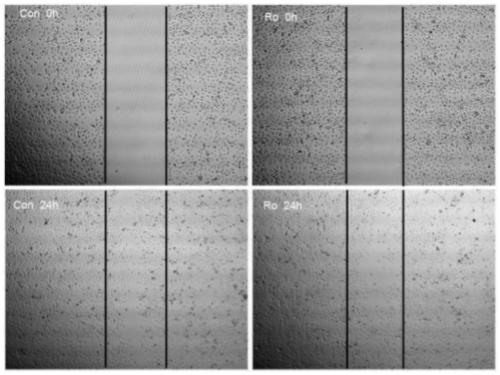
The invention relates to an application of ginsenoside Ro to preparation of a skin wound repair drug. The skin wound is caused by keratinocyte injury or caused by keratinocyte damage. The applicationhas advantages as follows: a HaCaT human keratinocyte experiment indicates that Ro has an obvious proliferative effect on HaCaT cells, and with the increase of time, the proliferation rate of the HaCaT cells tends to be reduced; and when concentration is increased gradually, the proliferation rate of the HaCaT cells is also increased. After ginsenoside Ro treatment is carried out for 16 h, it canbe observed under a light microscope that when the cells of a Ro treatment group are compared with the cells of a control group, the migration distances have no significant difference. After Ro treatment, the HaCaT G0 / G1 ratio is reduced, the S-phase cell ratio changes a little, and the G2 / M cell ratio is slightly increased, however, no obvious trend of time or dosage dependence is produced.
Owner:SHANGHAI CHANGHAI HOSPITAL
A method for tissue culture and rapid propagation using the bulb plate of Amaryllis chinensis as explants
InactiveCN109329060BReduce reproductionEfficient reproductionPlant tissue cultureHorticulture methodsPlantletAmaryllis
The invention provides a method for inducing adventitious buds to obtain regenerated plants by using the bulb disc of Amaryllis amaryllis as an explant, comprising the following steps: selection and disinfection of explants, induction of adventitious buds, subculture and multiplication, rooting Induction and transplantation of tissue culture seedlings. The method of the invention induces a germination rate of 98%, the proliferation of adventitious buds of the first generation single plant is more than 15 times, and the rooting rate reaches 99%, and the method can effectively solve the problem of in vitro rapid propagation of Lycoris amaryllis.
Owner:INST OF BOTANY JIANGSU PROVINCE & CHINESE ACADEMY OF SCI
A method for inducing adventitious buds using Lycoris safflower rachis as explants
ActiveCN106561450BReduce reproductionGet efficientlyPlant tissue cultureHorticulture methodsBudInflorescence
The invention provides a method for inducing adventitious buds by adopting Lycoris radiate rachis as explant in order to obtain regenerated plants. The method comprises the following steps: selection and disinfection of the explant, induction of the adventitious buds, subculture and propagation, root induction, and transplantation of tissue culture plants. The method allows the induction germination rate to reach 96%, 4.9 times or above adventitious buds to be proliferated from a per plant and the rooting rate to reach 94%, and can effectively solve the problem of in vitro rapid propagation of Lycoris radiate.
Owner:INST OF BOTANY JIANGSU PROVINCE & CHINESE ACADEMY OF SCI
Tissue Culture and Rapid Propagation Method of Fenza 1 Fenjiao
The invention discloses a Fenza No.1 dwarf banana tissue culture rapid propagation method, and belongs to the field of plant tissue culture. The method comprises the following steps: selecting a plant having green and stout cauloid, the height of 3.2-4.5 m, big fruit clusters, fewer fruit finger conjoined fruits, normal fruit shape and good quality as a mother plant; spraying absorptive buds on the mother plant with a carbendazim and streptomycin mixed liquid, 1 week later, taking the absorptive buds as explants, and inoculating; controlling a differentiation culture temperature to be 27 DEG C-33 DEG C, carrying out differentiation culture for 12-20 days, subculturing, and controlling the total number of subculture generations to be 12-16; and carrying out rooting culture and hardening seedlings. The Fenza No.1 rooting seedlings bred by the technology has more, stout and white root systems, pale yellow green plantlet cauloids, dark green leaves and high provisonal planting survival rate, and grow quickly and neatly; each explant can reproduce about 5000-8000 plantlets within a year, after the plantlets are fix-planted, the seed nature is stable, fewer variant plants appear, and banana farmers have good reflection.
Owner:POMOLOGY RES INST GUANGDONG ACADEMY OF AGRI SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com