Calycosin derivative as well as synthesis method and application thereof
A synthesis method and reaction technology, applied in the field of medicine, can solve problems such as unclear targets and large effective concentrations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
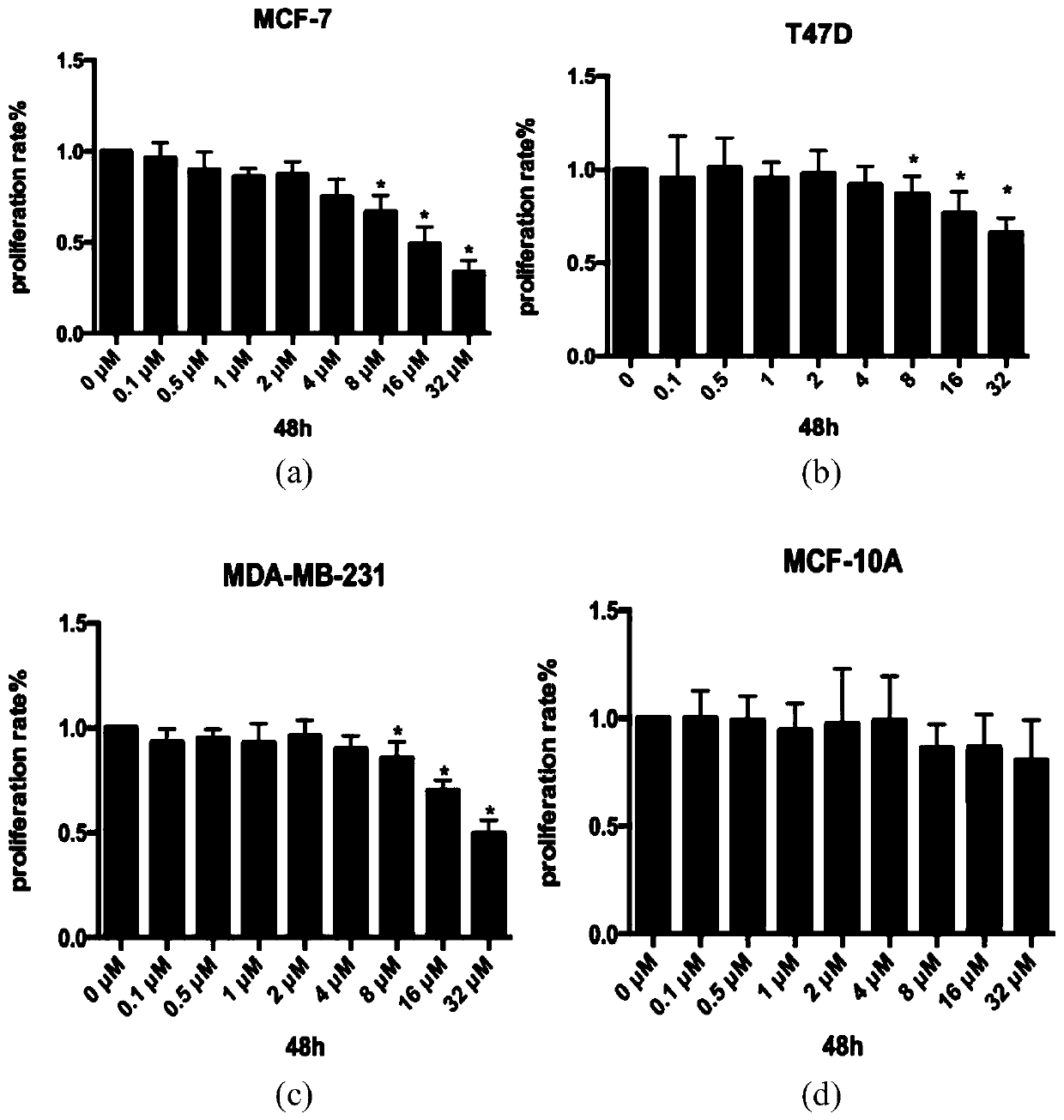
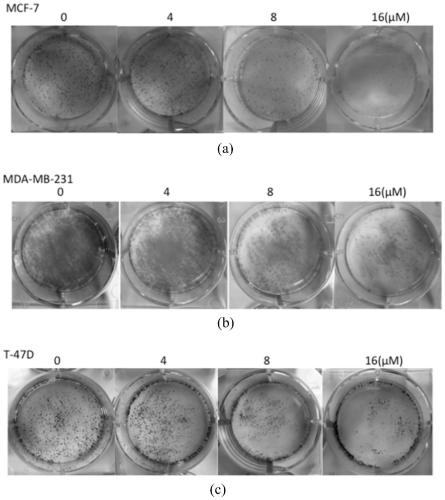
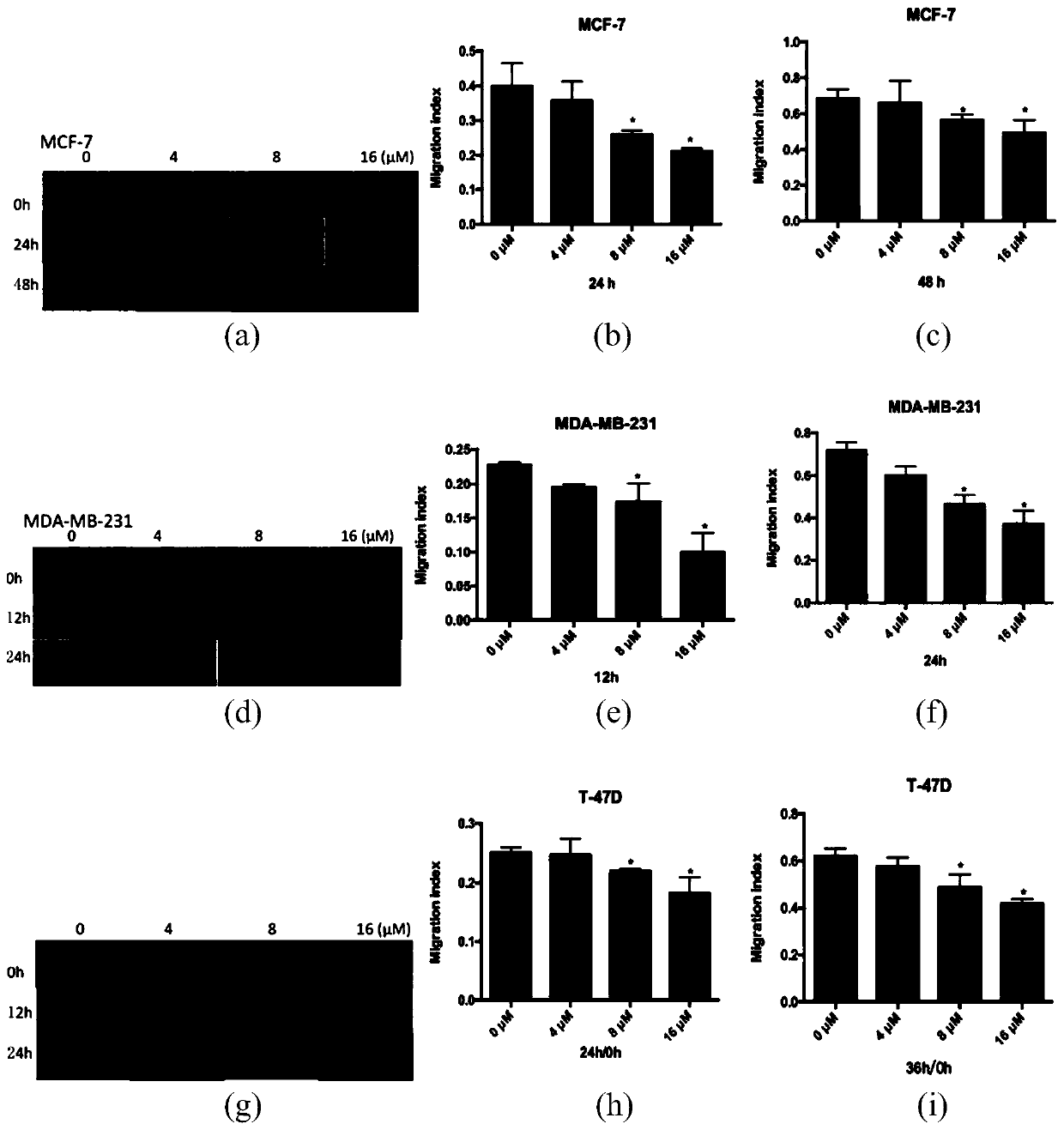
Image
Examples
Embodiment 1
[0039] Embodiment 1: Compound shown in synthetic formula (I) according to following synthetic route
[0040]
[0041] Among them, the chemical name of compound 1 is: 7-hydroxy-3-(3-hydroxy-4-methoxy)-4H-benzopyran-4-one, and compound 2 is ethyl chloroacetate.
[0042] The specific synthesis method is:
[0043] Take 1g (3.52mmol) of compound 1 (Caleoisoflavone) in a 50ml round bottom flask and dissolve it with 40ml of acetone, then add 3g of anhydrous K 2 CO 3 and 0.5gNaI, stirred at room temperature for 1h, then added dropwise 0.91g (7.43mmol) of compound 2 (ethyl chloroacetate), then placed in a 45°C water bath and stirred and refluxed for 4h (TCL to monitor the reaction, the developer was chloroform:methanol=60: 1, volume ratio); then stop heating and continue stirring until cooling. Add ice water to the cooled reactant, filter, collect the precipitate, dissolve the precipitate with ethyl acetate and dry with anhydrous sodium sulfate, evaporate the resulting solution t...
Embodiment 2
[0049] Embodiment 2: compound shown in synthetic formula (I)
[0050] Repeat Example 1, the difference is that with anhydrous Na 2 CO 3 instead of anhydrous K 2 CO 3 , replace NaI with KI, and change the reaction to 55°C.
[0051] Finally, a pale yellow solid was obtained, 70.21%.
[0052]The product obtained in this example was characterized by proton nuclear magnetic resonance spectrum and carbon spectrum, and was determined to be the compound shown in formula (I).
Embodiment 3
[0053] Embodiment 3: compound shown in synthetic formula (I)
[0054] Repeat Example 1, except that DMF is used instead of acetone and triethylamine is used instead of anhydrous K 2 CO 3 , and the reaction was carried out at 40°C.
[0055] Finally, a pale yellow solid was obtained, 23.18%.
[0056] The product obtained in this example was characterized by proton nuclear magnetic resonance spectrum and carbon spectrum, and was determined to be the compound shown in formula (I).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com