Preparation method of genetically modified T cells expressing chimeric antigen receptors
A chimeric antigen receptor, gene modification technology, applied in receptor/cell surface antigen/cell surface determinant, fusion cells, cells modified by introducing foreign genetic material, etc., can solve the problem of cell survival rate, cell proliferation rate Reduced, low gene transfer efficiency, cell damage, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
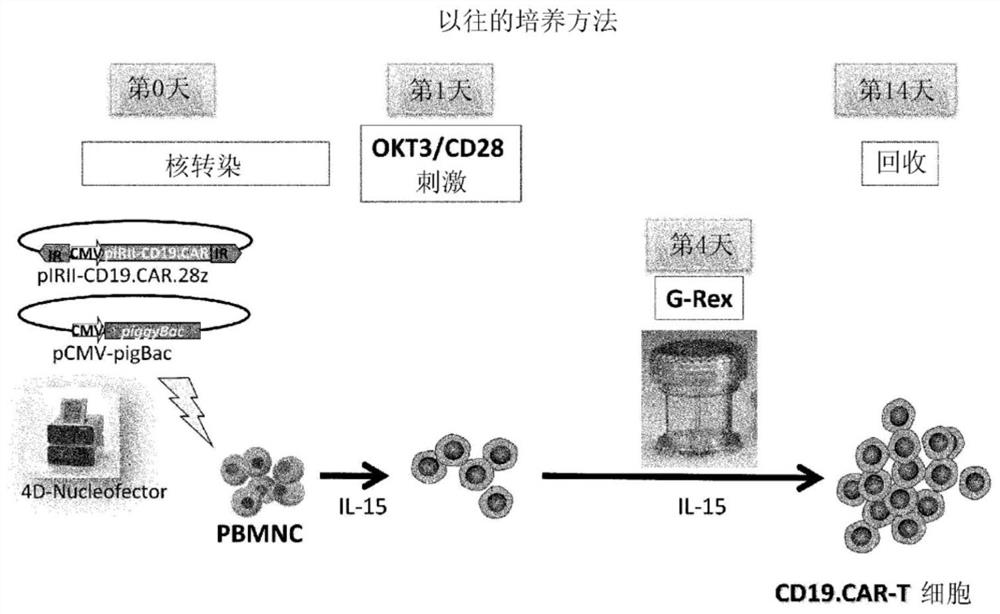
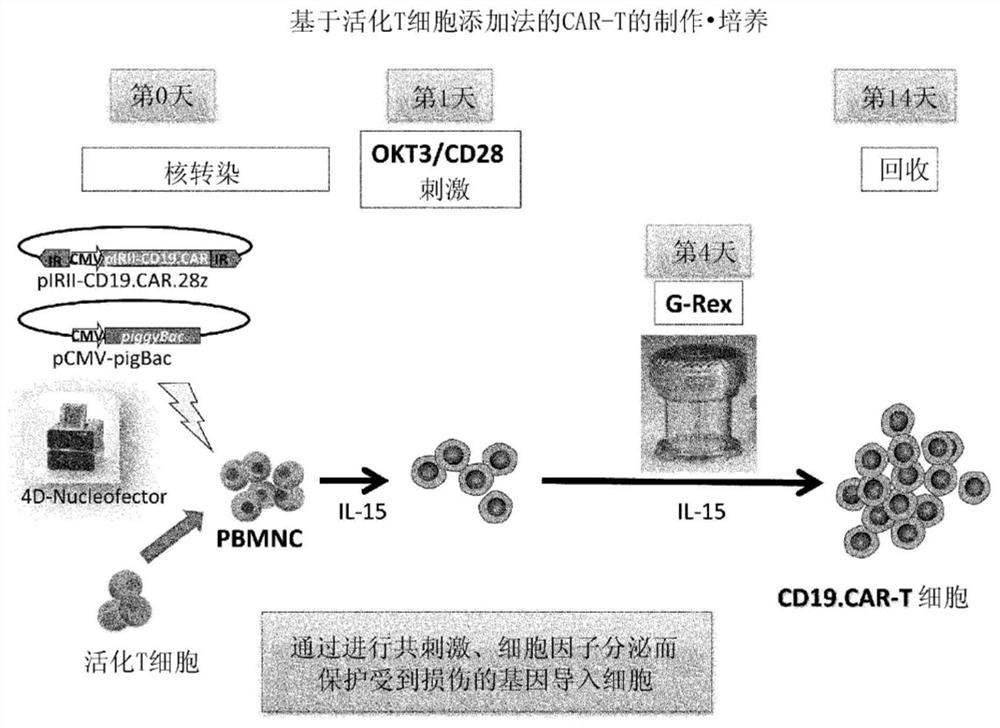
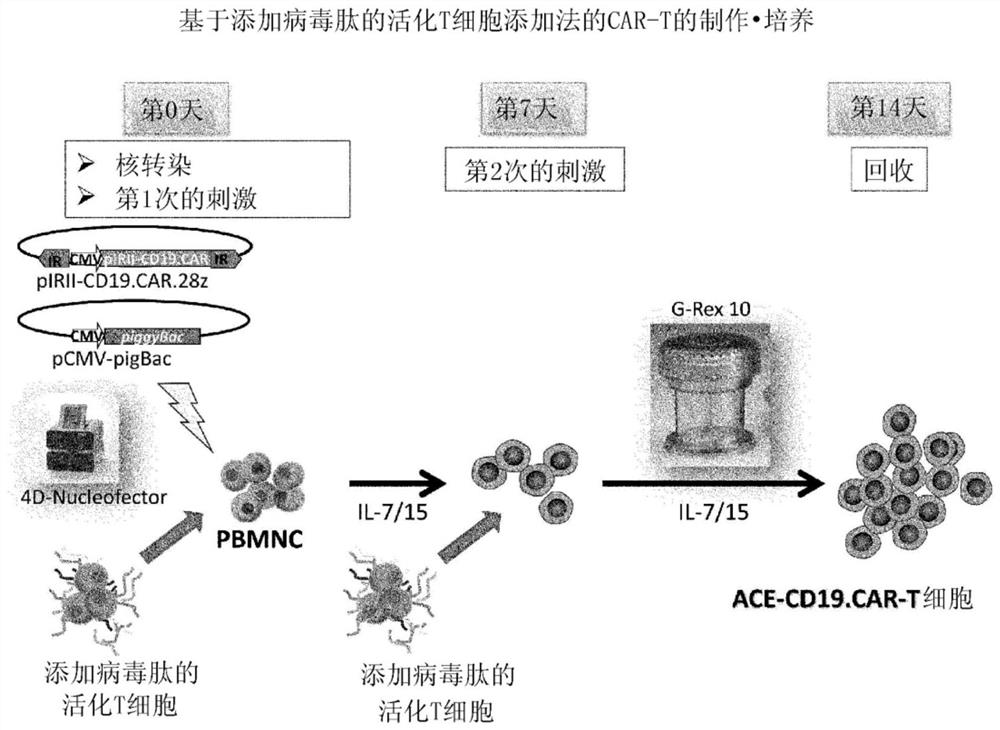
[0095] The preparation method of this mode includes the following steps (i) to (iv). In addition, for matters not mentioned (for example, the preparation method of the T cell-containing cell population, the basic manipulation based on stimulation with anti-CD3 antibody and anti-CD28 antibody, the method for the treatment to incapacitate the proliferation ability, the method based on the transposon) The operation of gene introduction, the basic operation of co-cultivation, the recovery method of cells, etc.) are the same as the above-mentioned first preparation method, therefore, the overlapping description is omitted, and the corresponding description is cited.
[0096] (i) Step of preparing non-proliferative cells holding viral peptide antigens by stimulating the T cell-containing cell population with anti-CD3 antibody and anti-CD28 antibody , obtained by culturing in the presence of viral peptide antigens and treatment to incapacitate the ability to proliferate
[0097] (ii...
Embodiment
[0115]
[0116] Compared with the case of using viral vectors, CAR therapy using the transposon method is particularly advantageous in terms of safety. On the other hand, the efficiency of gene introduction is low, the cells are easily damaged during the operation (for example, electroporation) at the time of gene introduction, and the number of obtained cells is small. In order to overcome these problems, the following studies were conducted.
[0117] 1. Materials
[0118] (1) Antibodies
[0119] Anti-CD3 antibody (Miltenyi Biotec)
[0120] Anti-CD28 antibody (Miltenyi Biotec)
[0121] (2) Culture medium
[0122] TexMACS (Miltenyi Biotec)
[0123] (3) Cytokine
[0124] Recombinant human IL-7 (Miltenyi Biotec)
[0125] Recombinant human IL-15 (Miltenyi Biotec)
[0126] (4) Viral peptide mixture
[0127] PepTivator (registered trademark) CMV pp65-premium grade, human (Miltenyi Biotec)
[0128] PepTivator (registered trademark) AdV5Hexon-premium grade, human (Milteny...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com