Mytilus crassitesta oligopeptide and use thereof
A thick-shelled mussel and oligopeptide technology, applied in the direction of peptide, peptide source, application, etc., can solve problems such as angioedema, rash, azotemia, death, etc., and achieve no cytotoxicity and high ACE inhibitory activity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0023] Embodiments of the present disclosure also provide a method for preparing mussel oligopeptides, including:
[0024] 1) Thaw the frozen thick-shelled mussel meat under running water at room temperature, clean it, dry the surface moisture at low temperature, dissect and mince it, add ethyl acetate to soak for 24-72 hours, stir continuously during the degreasing process, and vacuum filter the degreased sample After removing ethyl acetate (recovered by rotary steaming), dry and pulverize to obtain thick-shell mussel powder;
[0025] 2) According to the ratio of material to liquid: 1:20-60, add water to the thick-shelled mussel powder, then add 1-5% trypsin, enzymolyze at pH 7-9, 30-50°C for 3.0-5.0h, and the reaction is over Finally, inactivate the enzyme to obtain the enzymatic solution;
[0026] 3) Selecting an ultrafiltration membrane with a molecular weight cut-off of 1kDa to perform ultrafiltration on the mussel enzymatic hydrolyzate obtained in step 2) to obtain a <1...
Embodiment 1
[0032] A preparation method of mussel oligopeptide, comprising:
[0033] 1) Thaw the frozen thick-shell mussel meat under running water at room temperature, clean it, dry the surface moisture at low temperature, dissect and mince it, soak it in ethyl acetate for 48 hours, stir continuously during the degreasing process, and vacuum filter the degreased sample to remove acetic acid After the ethyl ester (recovered by rotary steaming), it is dried and pulverized to obtain thick-shelled mussel powder;
[0034] 2) Add water to the thick-shelled mussel powder according to the ratio of material to liquid 1:40, then add 3% trypsin, and enzymolyze it at pH 8 and 40°C for 4.0 hours, and heat it in boiling water at 100°C after the reaction Inactivate the enzyme for 10 minutes to obtain the enzymatic solution;
[0035] 3) Selecting an ultrafiltration membrane with a molecular weight cut-off of 1kDa to perform ultrafiltration on the mussel enzymatic hydrolyzate obtained in step 2) to obta...
Embodiment 2
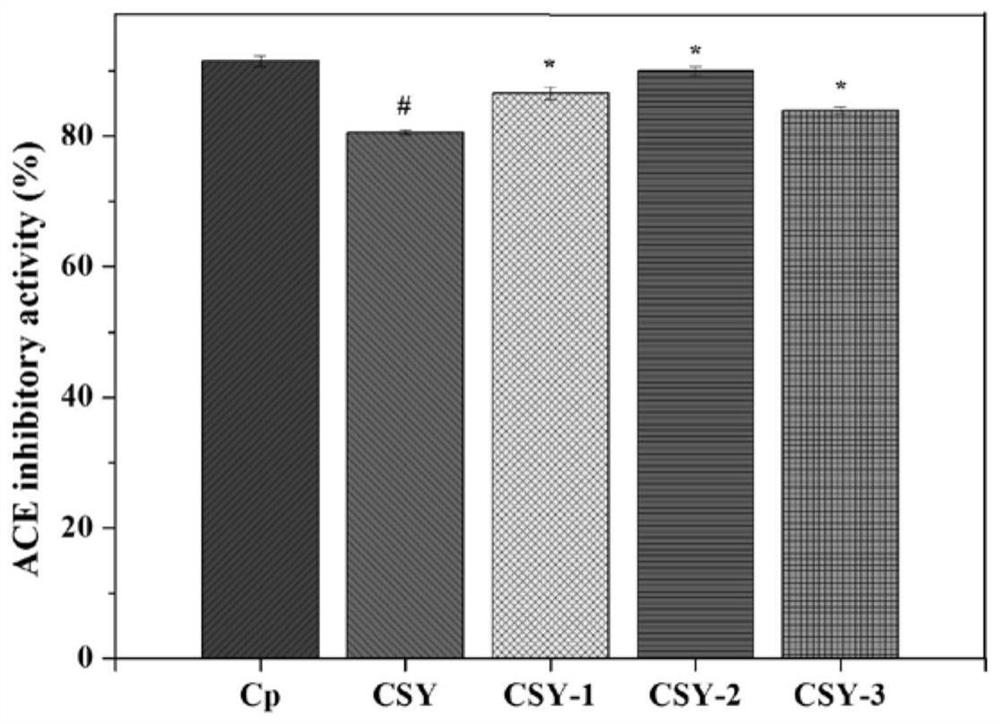
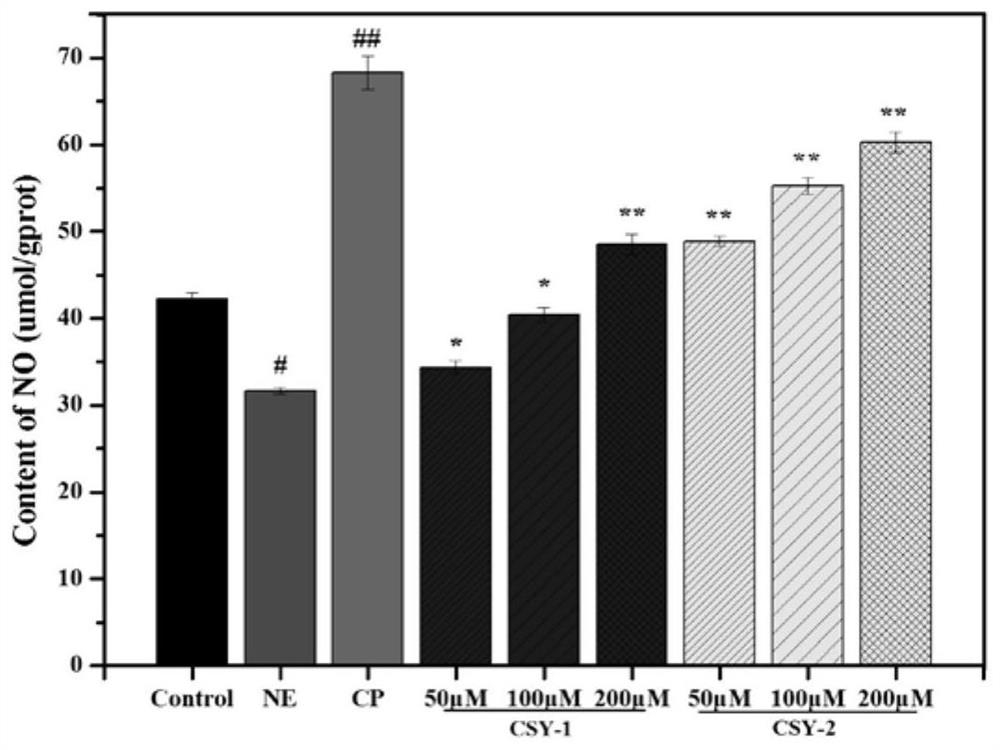
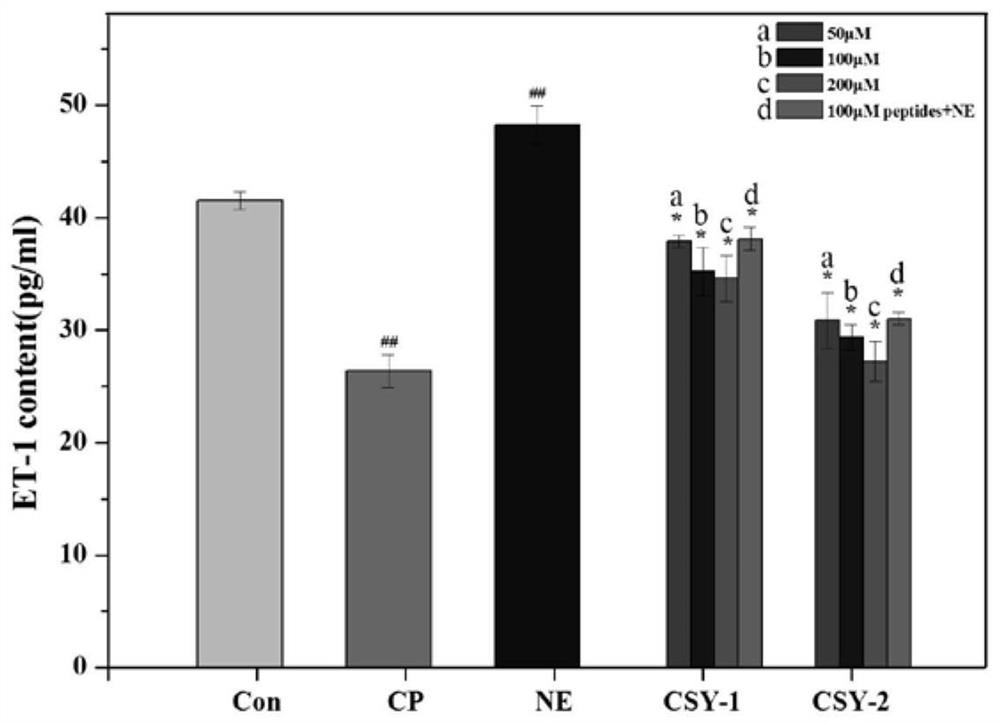
[0045] Test of ACE Inhibitory Activity of Oligopeptides from Mussel mussels
[0046] Main reagents: hydrochloric acid (analytical pure), sodium chloride (analytical pure), sodium hydroxide (analytical pure), purchased from Sinopharm Chemical Reagent Co., Ltd.; N-2-hydroxyethylpiperazine-N'-2-B Sulfonic acid (HEPES) was purchased from Solarbio; angiotensin-converting enzyme (ACE) and FAPGG (N-[3-(2-Furyl)acryloyl]-Phe-Gly-Gly) were purchased from Sigma, USA.
[0047] The preparation of reagent: 80mM HEPES buffer solution (pH 8.3, Cl - Concentration: 300mM): HEPES 1.910g, NaCl 1.755g, dissolved in double-distilled water, adjusted pH to 8.3 with NaOH, added water to 100mL, set at 4°C for later use; FAPGG solution (1mM): Take 3.994mg FAPGG powder and add HEPES buffer , mix and dissolve, dilute to 10mL, and store at 4°C in the dark; ACE inhibitor (peptide to be tested): weigh an appropriate amount of active peptide powder, and use HEPES buffer as a solvent to prepare a series of a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com