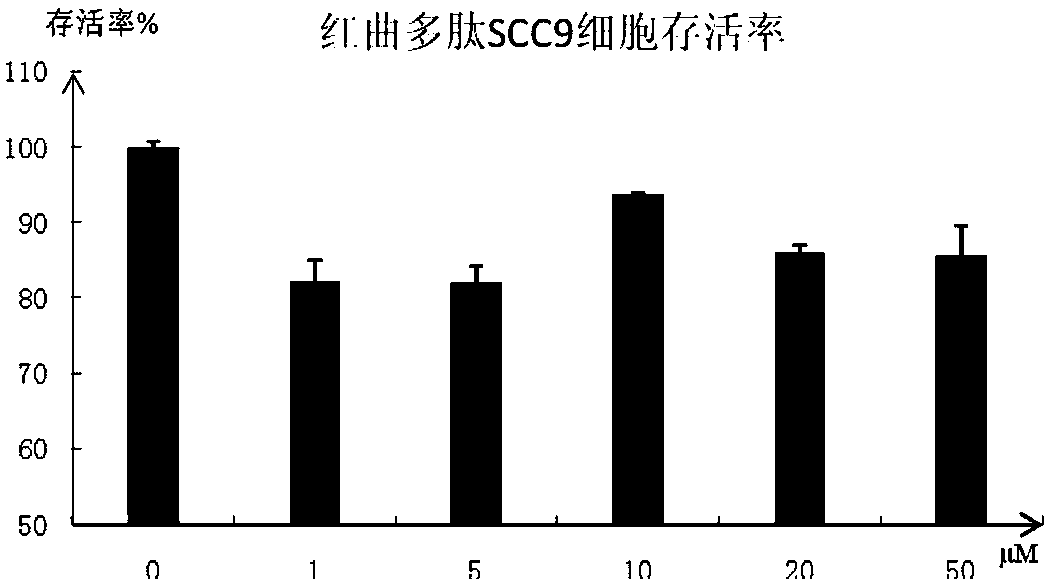
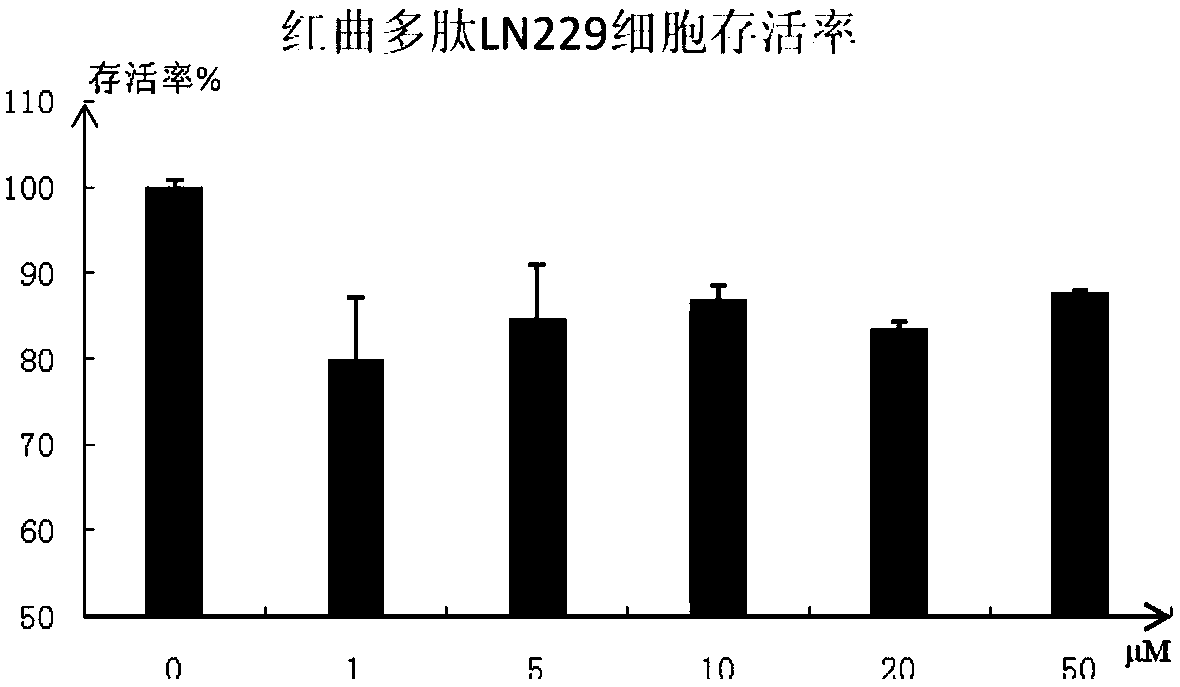
Polypeptide molecule with ACE (Angiotensin Converting Enzyme) inhibition activity and tumor resistance and preparation method thereof
A technology of inhibiting activity and anti-tumor, which is applied to the preparation method of peptides, anti-tumor drugs, chemical instruments and methods, etc. It can solve the problems of taste disorder and achieve the effect of small molecular weight, novel structure and high blood pressure-lowering activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031]The Monascus mycelium ACE inhibitory peptide dry powder obtained by vacuum freeze-drying was reconstituted in PBS buffer with a concentration of 10mmol / L and a pH of 7.3-7.5; centrifuge the reconstituted ACE inhibitory peptide solution at 3000r / min for 5min , collect the supernatant, and filter it with a 0.22-micron water-based microporous membrane. The obtained filtrate is separated by superdex 30pg Sephadex column chromatography of GE Company, the injection volume is 10mL, the column temperature is 25°C, and the mobile phase is superdex Pure water, flow rate 1mL / min, protein detection wavelength 280nm; collect Monascus mycelia ACE inhibitory peptide active component peaks, numbered as components A-E. Component E with the highest ACE inhibitory activity was centrifuged at 3000r / min for 5min, and the supernatant was collected and filtered through a 0.22 micron water-based microporous membrane. The resulting filtrate was further separated by RP-HPLC. The separation conditi...
Embodiment 2
[0035] The preparation method is the same as in Example 1.
[0036] Dissolve the sample in ultrapure water, centrifuge at 7000 rpm for 15 min, take 10 μL of supernatant and 40 μL of 0.78 mmol / L substrate (hippuryl-L-His-L-Leu dissolved in 0.05 mol / L boric acid buffer at pH 8.3 solution containing 0.3mol / L NaCl) at 37°C for 6min, add 20μL ACE (0.1U dissolved in 1.0mL of the same buffer solution), react at 37°C for 30min, add 80μL 1mol / L HCl solution to terminate the reaction , to room temperature, take 10 μL of the reaction product for detection. Its ACE inhibitory activity was measured.
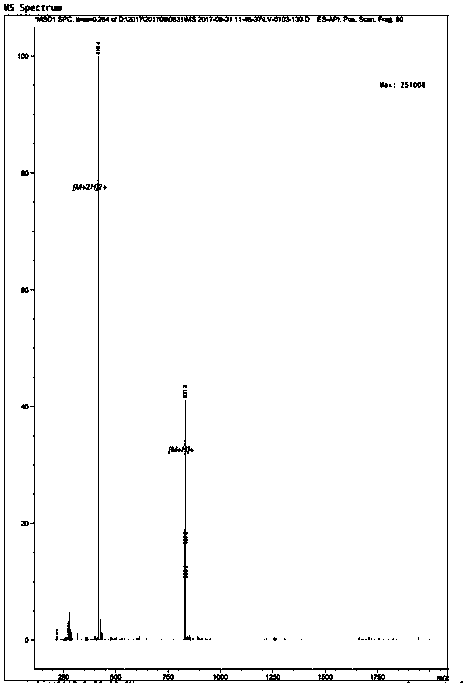
[0037] The results confirmed that the peptide was a heptapeptide product, the amino acid composition was Asn-Thr-Met-Met-Thr-His-Pro, the molecular weight was 814.493 Da, and the half inhibitory concentration was 0.18 mg / mL.
Embodiment 3
[0039] Using 2-Chlorotrityl Chloride Resin as the starting material, follow the method of solid-phase synthesis to connect sequentially with fluorenylmethoxycarbonyl (Fmoc-) as the protecting group Fmoc-L-Asn(trt)-OH, Fmoc-L-Met- OH, Fmoc-L-Thr(Tbu)-OH, Fmoc-L-His(Trt)-OH, Fmoc-L-Pro-OH, the reaction was carried out at room temperature to obtain a heptapeptide resin, during which DIEA and HBTU were used in turn as Condensing agent, 20% piperidine DMF as deprotection agent for peptide grafting reaction, single amino acid peptide grafting reaction time 2h, deprotection time 20min, to obtain heptapeptide compound crude product, and then through reverse phase C18 column purification peptide, freeze-dried, obtained The ACE-inhibiting heptapeptide product. The obtained product will be collected and sampled for identification of purity and MS.
[0040] Dissolve the sample in ultrapure water, centrifuge at 7000 rpm for 15 min, take 10 μL of supernatant and 40 μL of 6.5 mmol / L substra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com