Preparation and refining methods of dasatinib
A dasatinib and refining method technology, which is applied in the new preparation and refining field, can solve the problems of cumbersome refining steps in the refining method, harsh requirements on the reaction vessel, and deterioration of dasatinib, and achieve simple operation, good stability, short-term effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
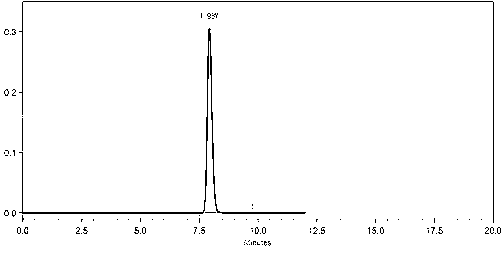
Image
Examples
Embodiment 1
[0050] Preparation and purification of dasatinib
[0051] Preparation of Dasatinib
[0052]Dissolve 100g of N-(2-chloro-6-methylphenyl)-2[(6-chloro-2-methyl-4-pyrimidinyl)amino]-5-thiazolecarboxamide in 300ml of dimethylsulfoxide Add 98.9g of 1-(2-hydroxyethyl)piperazine and 65.5g of diisopropylethylamine respectively, heat at 80°C, stir for 2-3h, monitor by HPLC, stop the reaction until the raw materials disappear completely. Naturally cooled to room temperature, 900ml of isopropanol was added to the reaction solution, solids were precipitated, cooled and stirred for 2h. After the precipitation was complete, it was filtered, and the filter cake was washed successively with a small amount of isopropanol and water to obtain 101.2 g of crude dasatinib. The calculated yield was 78.8%.
[0053] Refining of Dasatinib
[0054] Mix and dissolve the crude dasatinib obtained in the above experiment with 95% ethanol-water (2L: 0.4L), heat to 70°C, filter while hot after dissolution...
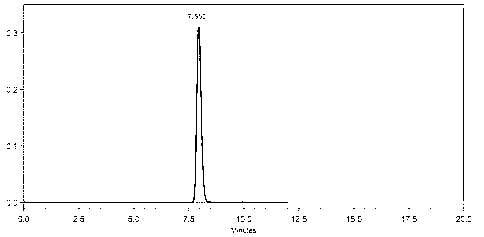
Embodiment 2
[0066] Investigation on drying conditions of dasatinib
[0067] Weigh 120g of crude dasatinib, heat it to 75°C with 95% ethanol-water (2L: 0.4L), and filter it while it is hot after the dissolution is complete. Cool the filtrate to room temperature, freeze and crystallize. Filter, wash with a small amount of cold 50% ethanol-water (1:1 / v:v), and dry at 50°C under normal pressure for 3 hours to obtain 90.4g of white solid with a yield of 75.3%, crushed and sieved, and weighed accurately Take 10.0g of each 8 parts in total, and dry them under vacuum at 105°C. See Table 1 for the drying time.
[0068] Table 1 Investigation of drying conditions of dasatinib
[0069] serial number temperature (°C) pressure time (h) Moisture content (%) color 1 0 3.66 White 2 105 vacuum 2 0.70 White 3 105 vacuum 4 0.38 White 4 105 vacuum 6 0.36 White 5 105 vacuum 8 0.33 White 6 105 vacuum 10 0.31 White 7 ...
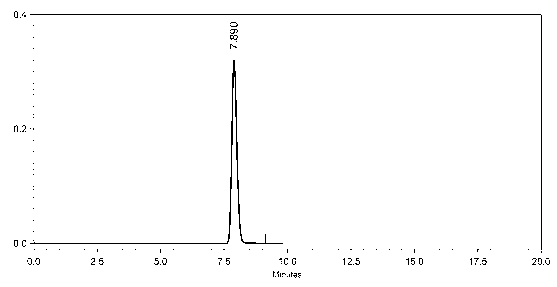
Embodiment 3
[0072] Preparation and purification of dasatinib
[0073] Preparation of Dasatinib
[0074] Dissolve 100g of N-(2-chloro-6-methylphenyl)-2[(6-chloro-2-methyl-4-pyrimidinyl)amino]-5-thiazolecarboxamide in 500ml hexamethylphosphoramide (HMPA), add 165.1g of 1-(2-hydroxyethyl)piperazine and 65.5g of diisopropylethylamine respectively, heat to 75°C, stir for 2-3h, monitor by HPLC until the raw materials disappear completely, stop the reaction, After cooling to room temperature, 1500 ml of isopropanol was added to the reaction liquid, and solids were precipitated, cooled and stirred for 2 h. After the precipitation was complete, it was filtered, and the filter cake was washed with a small amount of isopropanol and water in sequence to obtain 98.2 g of crude product. Yield: 76.5%.
[0075] Refining of Dasatinib
[0076] Mix and dissolve the crude product with 95% ethanol-water (2L: 0.4L), heat to 80°C, filter while hot after dissolution, cool the filtrate to room temperature...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com