Thiazole heterocyclic compounds, and preparation method and application thereof
A compound and heterocyclic technology, applied in the fields of thiazole heterocyclic compounds and their preparation and application, can solve the problems of poor curative effect of leukemia and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0085] Example 1 Chemical Synthesis and Structure Identification of Thiazole Heterocyclic Compounds with General Formula One
[0086]
[0087] In the above general formula one:
[0088]
[0089] The chemical synthesis experimental steps of the thiazole heterocyclic compounds with general formula one (the experimental steps of the example compounds included in general formula one) are as follows:
[0090] 1. General synthetic route
[0091]
[0092] 2. Specific synthesis steps:
[0093] 2.1: Compound 3: N-(2-chloro-6-methylphenyl)-2-((6-(4-(2-(1,3-dioxoisoindoline)ethyl)piperazine Synthesis of )-2-methylpyrimidine)amino)thiazole-5-carboxamide
[0094]
[0095] At 0°C, compound 1 (500mg, 1.02mmol), triphenylphosphine PPh 3 (349mg, 1.33mmol), phthalimide 2 (195.7mg, 1.33mg) was added to 10mL redistilled anhydrous tetrahydrofuran, and then added diisopropyl azodicarboxylate DIAD ( 226.4mg, 1.33mmol), stirred at low temperature for 10min and then moved to room temp...
example 1
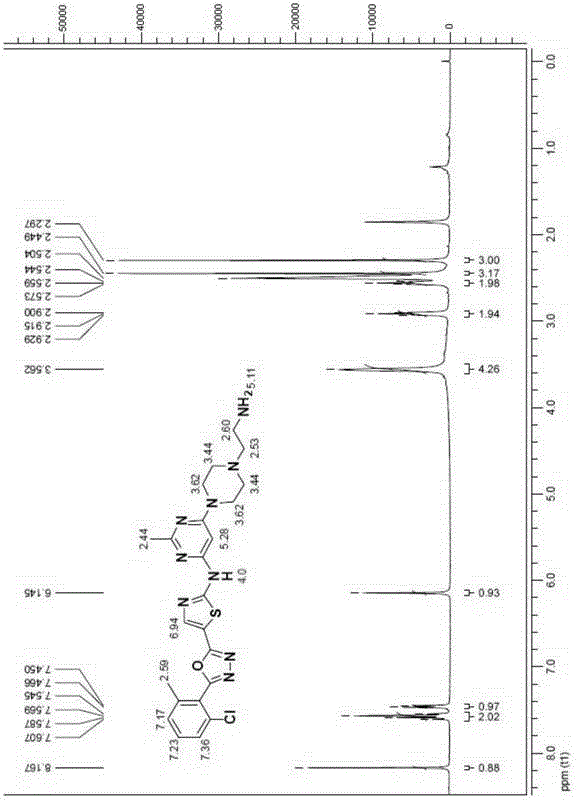
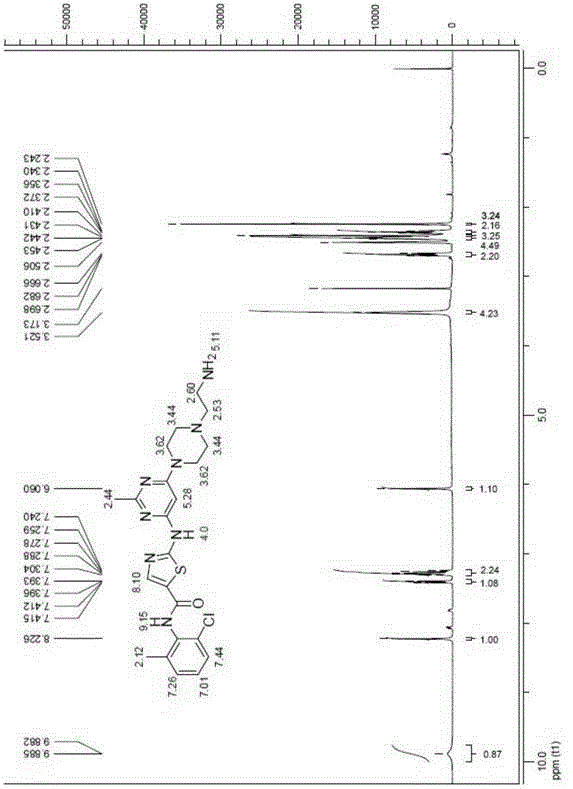
[0096] Example 1 compound 4a: N-(2-chloro-(6-methylphenyl)-2-[[6-[4-(2-aminoethyl)-1-piperazinyl]-2-methyl- Synthesis of 4-pyrimidinyl]amino]-5-thiazolecarboxamide
[0097]
[0098] Add compound 3 (606 mg, 0.98 mmol) into a round bottom flask with 10 mL of ethanol, then add 5 mL of hydrazine hydrate, heat to 78 ° C and reflux for two hours to complete the reaction, concentrate under reduced pressure to remove the reaction solvent, and then add chlorinated The sodium-saturated solution was extracted three times with tetrahydrofuran, and the organic phase was dried over anhydrous sodium sulfate, filtered, and concentrated. Further purification by column chromatography yielded compound 4a (white solid). 1HNMR (400MHz, DMSO-d 6 )δppm 9.88 (br, 1H), 8.23 (s, 1H), 7.40 (dd, J=7.2Hz, 1H), 7.24-7.30 (m, 2H), 6.06 (s, 1H), 3.52 (br, 4H ), 2.68(t, J=6.4Hz, 2H), 2.44(t, J=4.4Hz, 4H), 2.41(s, 3H), 2.36(t, J=6.4Hz, 2H), 2.24(s, 3H ); 13 CNMR (100MHz, DMSO-d 6 )δ165.61, 163.11, 1...
example 2
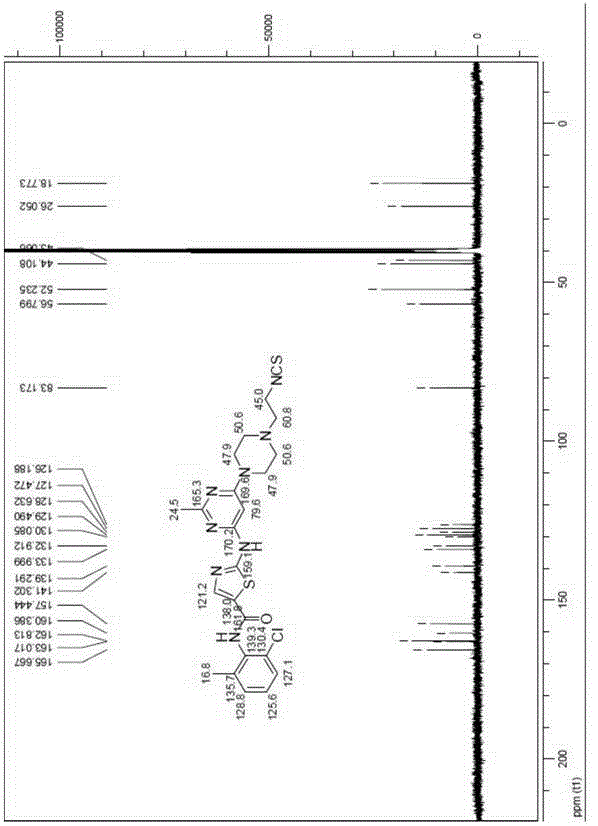
[0100] Example 2 Compound 4b: N-(2-chloro-(6-methylphenyl)-2-[[6-[4-(2-isothiocyanate)-1-piperazinyl]-2-form Synthesis of yl-4-pyrimidinyl]amino]-5-thiazolecarboxamide
[0101]
[0102] At 0°C, compound 4a (100mg, 0.21mmol) was added to a round bottom flask with 3mL of dichloromethane, then dicyclohexylcarbodiimide DCC (1.1eq) was added, and then carbon disulfide CS was slowly added dropwise under stirring. 2 (15eq), after the dropwise addition, stirred at low temperature for 5 minutes, removed the ice-water bath, and then stirred at room temperature until the reaction was complete. Concentrate under reduced pressure to remove the reaction solvent, and purify by silica gel column chromatography to obtain compound 4b (white solid). of the compound 1 HNMR (400MHz, DMSO-d 6 )δppm11.47(s, 1H), 9.88(s, 1H), 8.23(s, 1H), 7.40(dd, J=1.2, 7.2Hz, 1H), 7.24-7.30(m, 2H), 6.07(s , 1H), 3.80(t, J=6.0Hz, 2H), 3.54(br, 4H), 2.66(t, J=6.0Hz, 2H), 2.54(br, 4H), 2.42(s, 3H), 2.24 (s, 3H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com