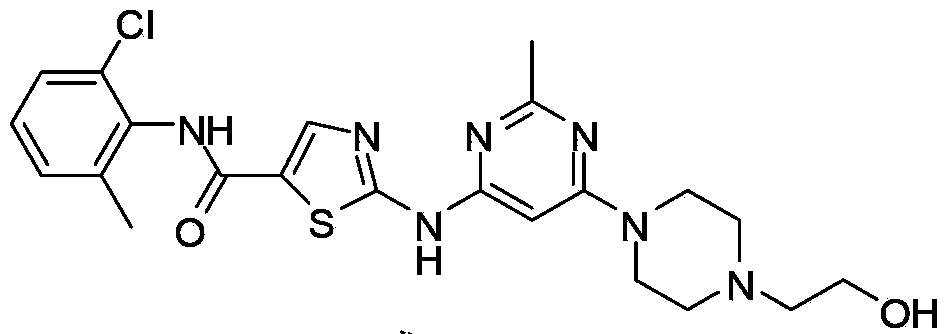
New Dasatinib crystal form and preparation method thereof
A dasatinib and crystal form technology, applied in the field of dasatinib-2-[[6-[4--1-piperazine, can solve the problems of no novelty and no application value, and achieve reproducibility Good performance, satisfying large-scale industrial production, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
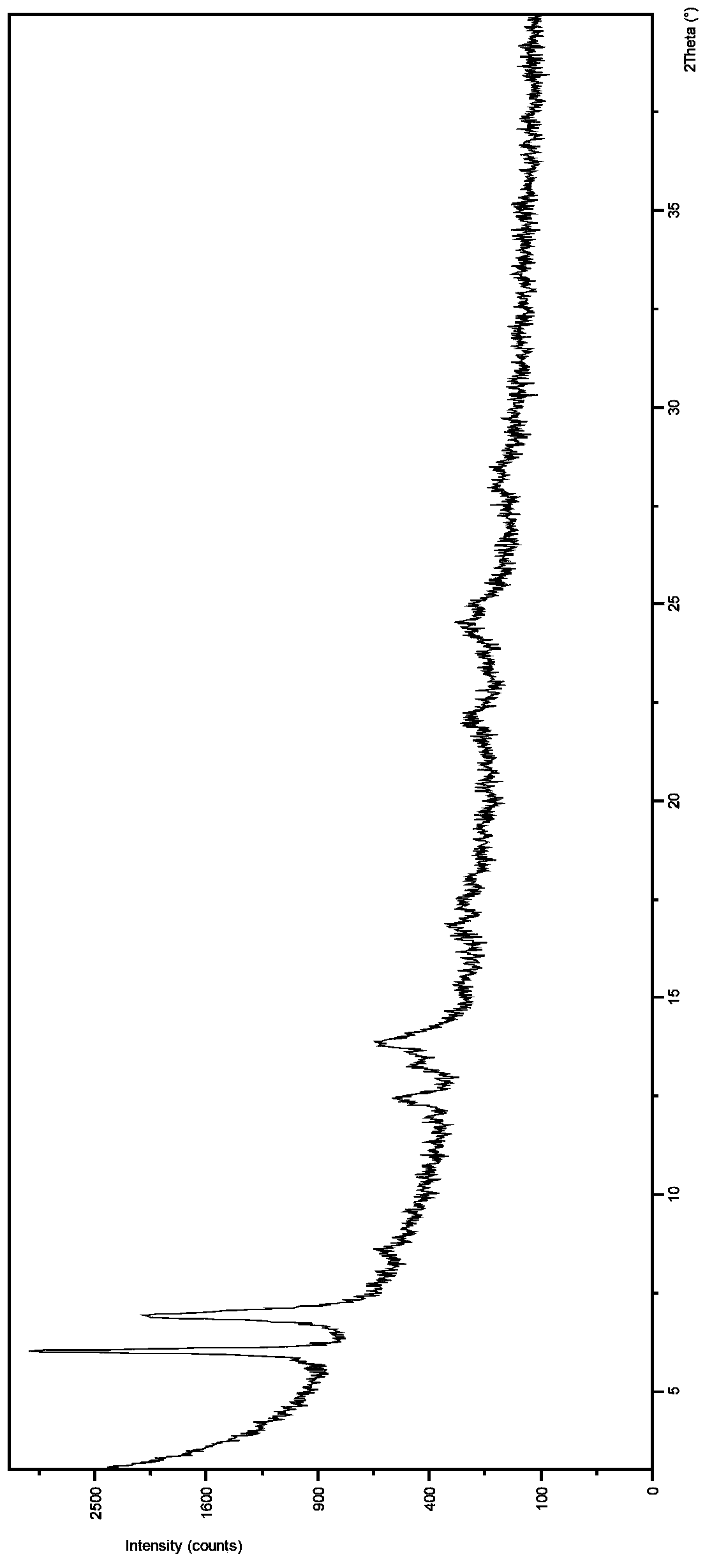
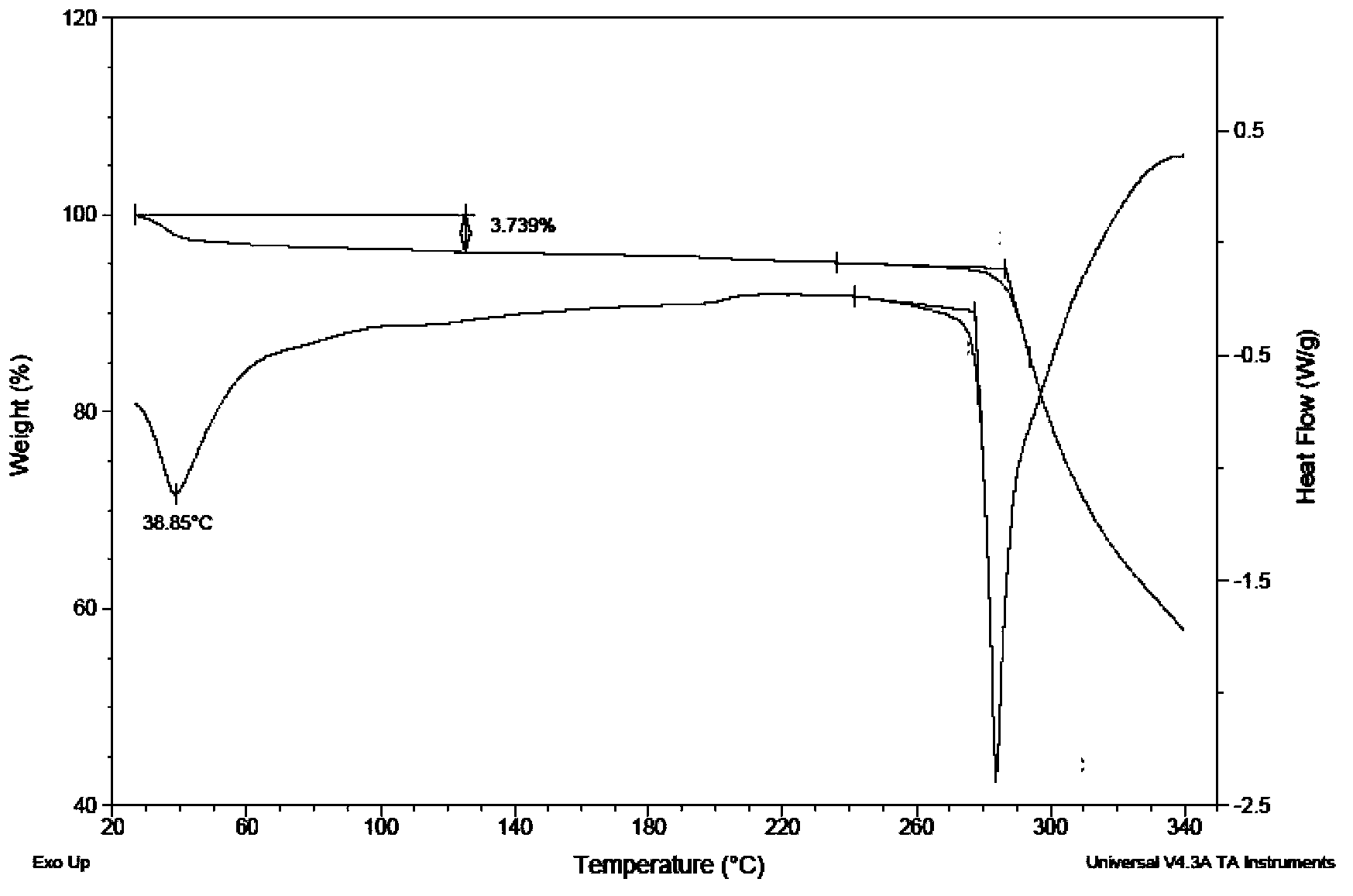
[0037] At room temperature, weigh 200mg of dasatinib free base, add it to 4ml of methanol solution, stir and suspend dasatinib in the solution system, add 1ml of 0.5M hydrochloric acid methanol solution dropwise to the suspension, and continue stirring until reaching Satinib is completely dissolved, and the filtrate is filtered for later use. Add 6ml of acetonitrile dropwise to the filtrate. After dripping, the solution is still clear. Then add 1ml of methanol containing 20mg of sodium hydroxide dropwise to the solution. After stirring for 5min, a solid precipitates out, continue stirring After 10 hours, filter and dry to obtain the new crystal form V of dasatinib.
Embodiment 2
[0039] At room temperature, weigh 200mg of dasatinib free base, add it to 4ml of ethanol solution, stir and suspend dasatinib in the solution system, add 1ml of 0.5M hydrochloric acid ethanol solution dropwise to the suspension, and continue stirring until reaching Satinib is completely dissolved, and the filtrate is filtered for later use. Add 6ml of acetonitrile dropwise to the filtrate. After dropping, the solution is still clear. Then add 1ml of ethanol containing 20mg of sodium hydroxide dropwise to the solution. After stirring for 5 minutes, solids precipitate out, and continue stirring. After 10 hours, filter and dry to obtain the new crystal form V of dasatinib.
Embodiment 3
[0041] At room temperature, weigh 200mg of dasatinib free base, add it to 5ml of isopropanol, stir and suspend dasatinib in the solution system, add 1ml of 0.5M isopropanol hydrochloride solution to the suspension, and continue stirring Until Dasatinib is completely dissolved, filter the filtrate for later use, add 6ml of ethyl acetate dropwise to the filtrate, after the drop, the solution is still clear, then add 1ml of isopropanol containing 20mg of sodium hydroxide dropwise to the solution, and stir for 5min Solids were precipitated, and the stirring was continued for 10 h, filtered and dried to obtain the new crystal form V of dasatinib.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com