Treatment of Restenosis and Stenosis with Dasatinib
a technology of dasatinib and restenosis, which is applied in the field of prevention and treatment of artery obstructive diseases, can solve the problems of renarrowing (restenosis) and limit the long-term efficacy of these revascularization therapies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
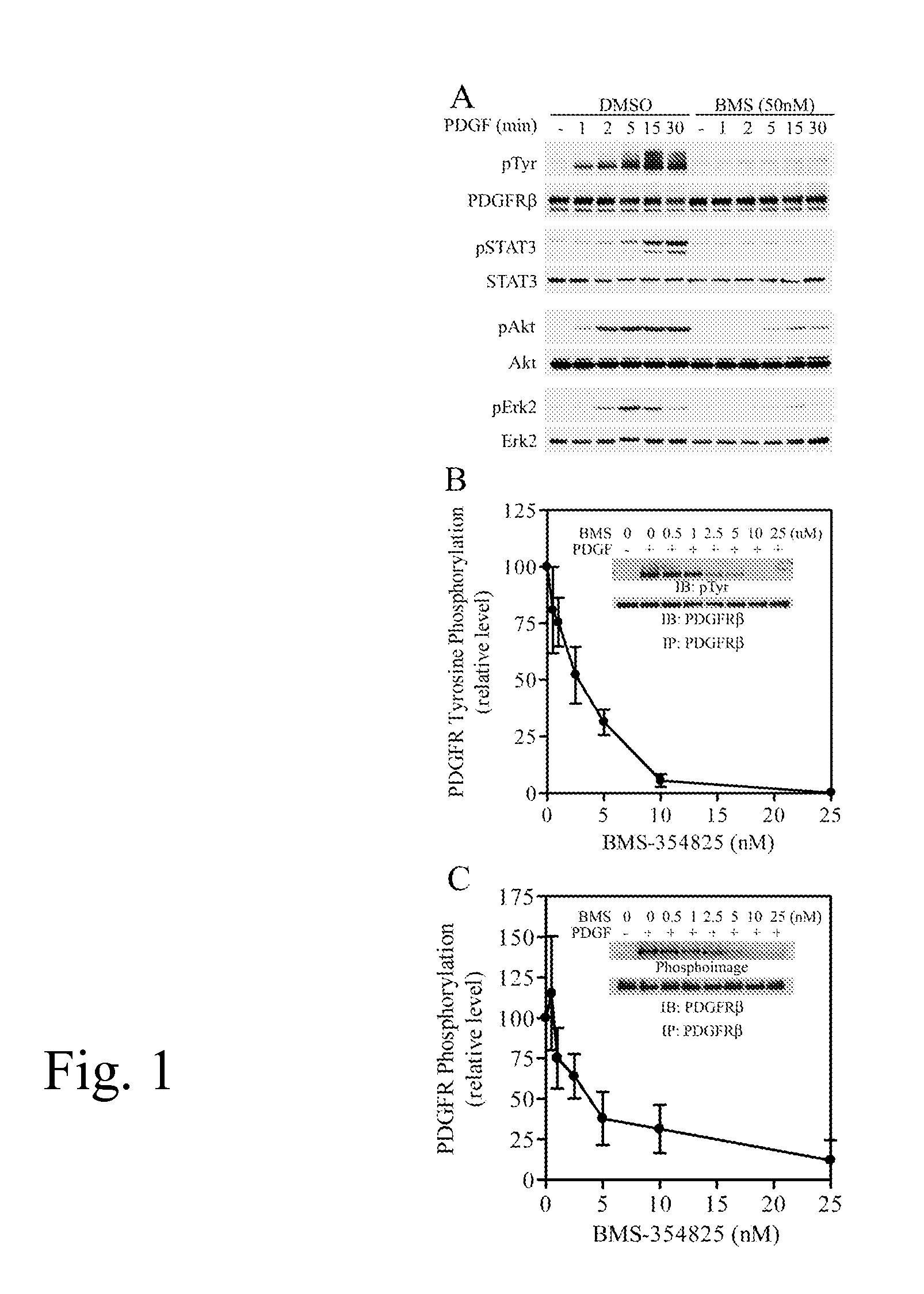
[0061] BMS-354825 Inhibits PDGF-activated Signaling Pathways in VSMCs.
[0062] To determine if BMS-354825 could inhibit PDGFR activation in VSMCs, we treated serum-starved rat A10 cells with PDGF-BB (5 ng / ml) for 0-30 min in the presence or absence of 50 nM BMS-354825. Cell lysates were analyzed for activation of PDGFRβ, STAT3, Akt, and Erk2 by immunoblotting analyses (FIG. 1A). PDGFRβ is the predominant PDGFR subunit in VSMCs (Raines, 2004) and the only functional PDGFR subunit in A10 cells (Rao et al., 1997). STAT3, Akt, and Erk2 signaling pathways have been reported to mediate PDGF-induced cell migration and proliferation in VSMCs (Graf et al., 1997; Kim et al., 2005; Neeli et al., 2004; Shibata et al., 2003; Vantler et al., 2005; Zhan et al., 2003). PDGF treatment markedly increased PDGFRβ tyrosine phosphorylation, which was detectable at the earliest time point examined (1 min). BMS-354825 (50 nM) effectively blocked PDGF-stimulated PDGFRβ tyrosine phosphorylation at all time po...
example 2
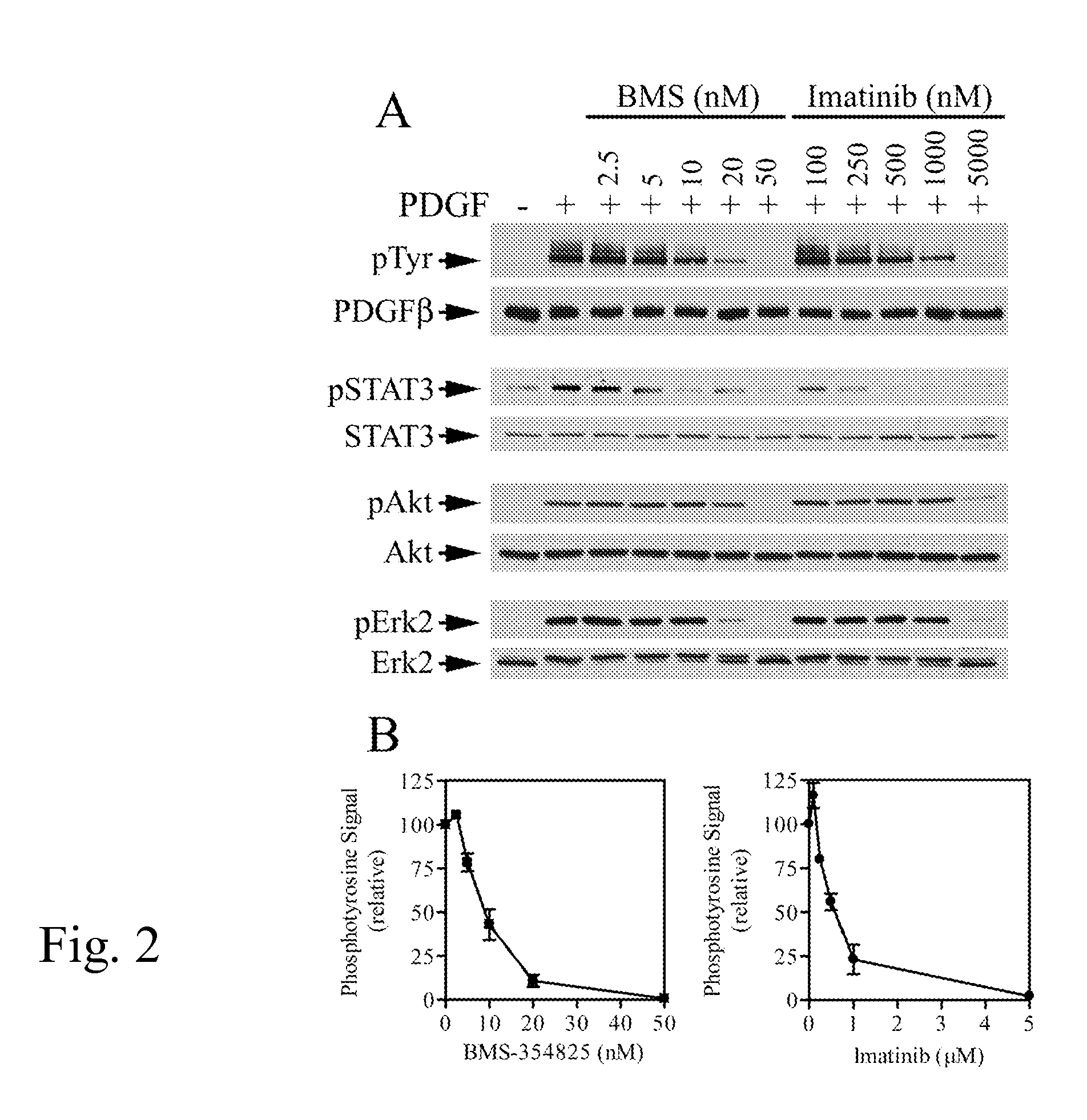
[0065] Comparison of PDGFR Inhibition by BMS-354825 and Imatinib.
[0066] In addition to inhibiting Bcr-Abl and c-Kit, imatinib also inhibits PDGFR tyrosine kinase. To compare the inhibition of PDGFR in VSMCs by BMS-354825 and imatinib, A10 cells were pre-incubated with various concentrations of BMS-354825 or imatinib, stimulated with PDGF-BB, and activation of PDGFR, STAT3, Akt, and Erk2 were analyzed. FIG. 2A shows that PDGF-stimulated PDGFRβ tyrosine phosphorylation was completely blocked by 50 nM BMS-354825. In contrast, 5 μM imatinib was required to achieve the same level of PDGFR inhibition in parallel experiments. Similarly, complete inhibition of PDGF-stimulated Akt and Erk2 activation were observed in cells treated with 50 nM BMS-354825 but only with 5 μM imatinib, whereas decrease in STAT3 tyrosine phosphorylation appeared at lower concentrations of imatinib (FIG. 2A). Of note, inhibition of Akt by both BMS-354825 and imatinib was observed only at the highest drug concentra...
example 3
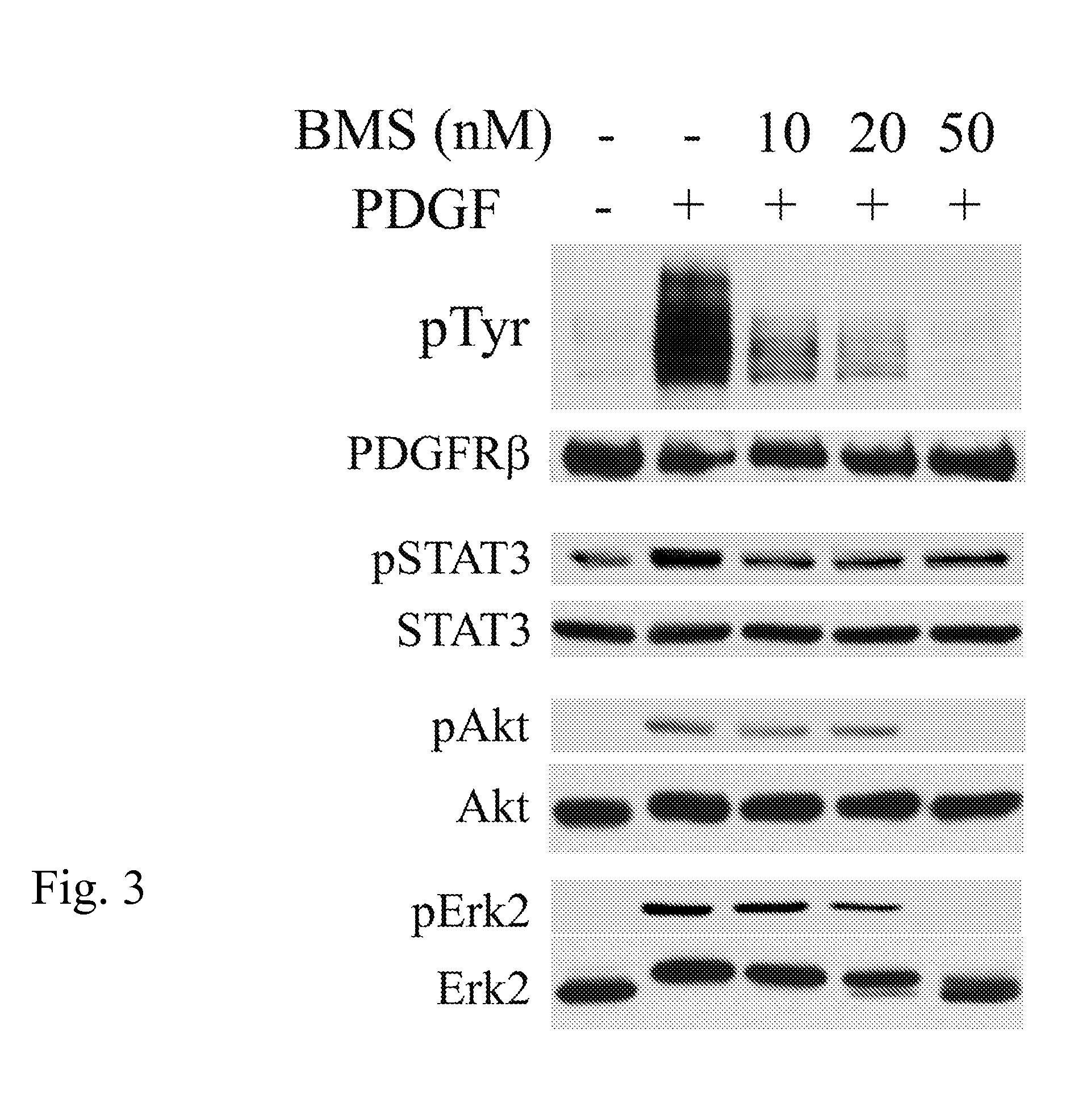
[0067] Inhibition of PDGFR in Human AoSMCs by BMS-354825.
[0068] To exclude the possibility that the potent inhibition of PDGFR by BMS-354825 is specific to rat VSMCs, we examined the effect of BMS-354825 in primary cultures of human AoSMCs. Human AoSMCs were pre-incubated with 0-50 nM BMS-354825 and then stimulated with PDGF-BB (5 ng / ml, 5 min). PDGFR tyrosine phosphorylation and activation of STAT3, Akt, and Erk2 were analyzed. PDGF markedly induced PDGFR tyrosine phosphorylation in human AoSMCs (FIG. 3). Similar to that observed in A10 cells, the PDGF-stimulated PDGFR tyrosine phosphorylation was completely blocked by 50 nM BMS-354825.
[0069] The primary culture of human AoSMCs appeared to have an elevated level of active STAT3 in the absence of PDGF stimulation (FIG. 3). Nevertheless, STAT3 was further activated by PDGF, which was blocked by BMS-354825. BMS-354825, however, was unable to reduce the active STAT3 to a level below the basal activation state in human AoSMCs. Similar...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentrations | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com