Medicinal dasatinib composition and preparation method thereof
A technology of dasatinib and a composition, applied in the field of pharmaceutical preparations, can solve problems such as unfavorable industrial production, complex process, long cycle, etc., and achieve the effects of avoiding unequal risks, improving microenvironment, and improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1. Dasatinib Tablets Prescription Composition: The specification is 70 mg (calculated as Dasatinib), and the prescription quantity is 1000 tablets.
[0050]
[0051]
[0052] Preparation method: mix the prescription amount of dasatinib anhydrous with lactose, microcrystalline cellulose, and hydroxypropyl methylcellulose evenly, add 90 g of purified water to hydroxypropyl cellulose to make a solution, and add the solution to the above-mentioned The dasatinib mixture was granulated by a high-shear wet granulator, dried, and then the prescribed amount of croscarmellose sodium and magnesium stearate was added, mixed evenly, and compressed into tablets.
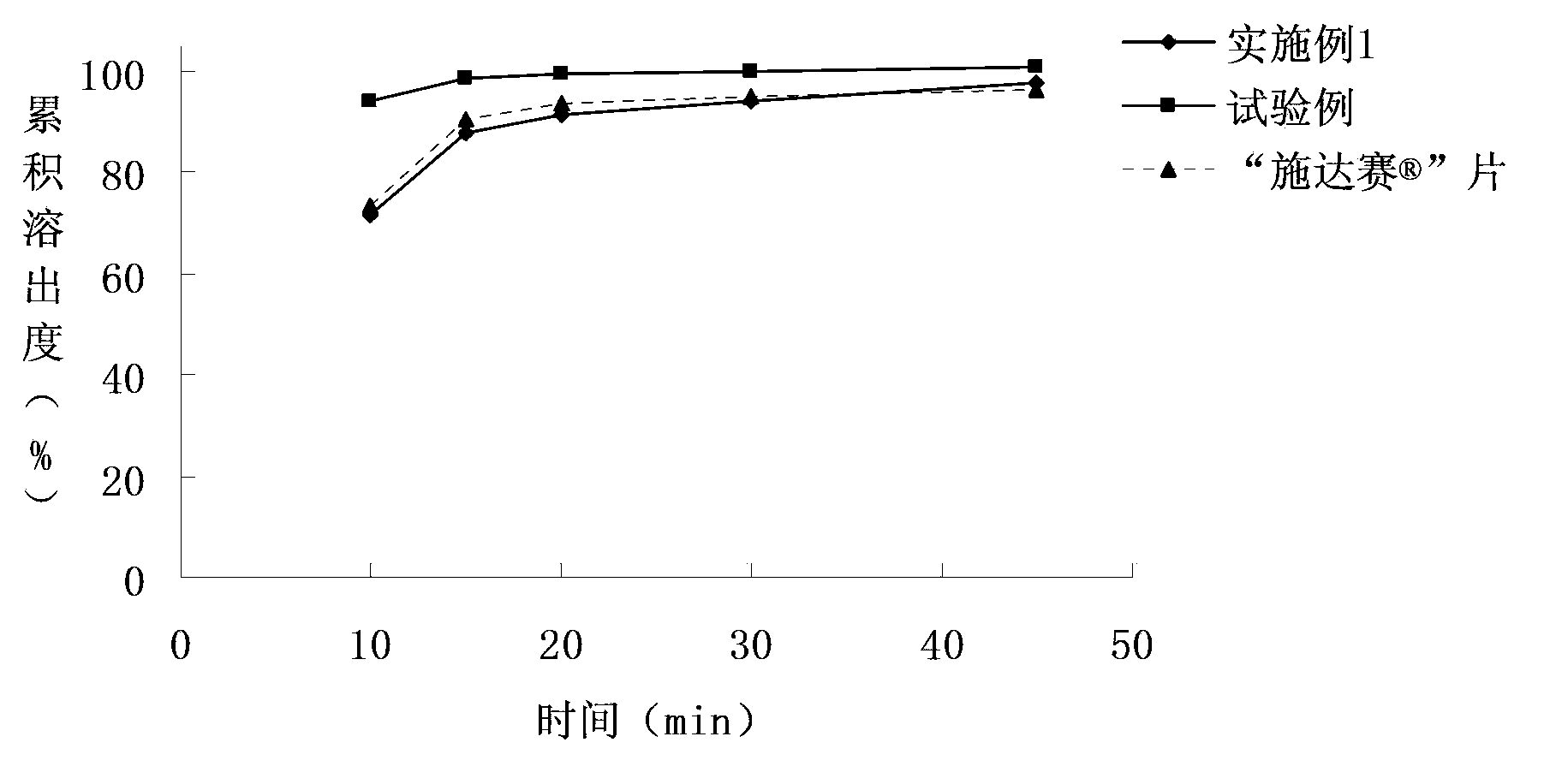
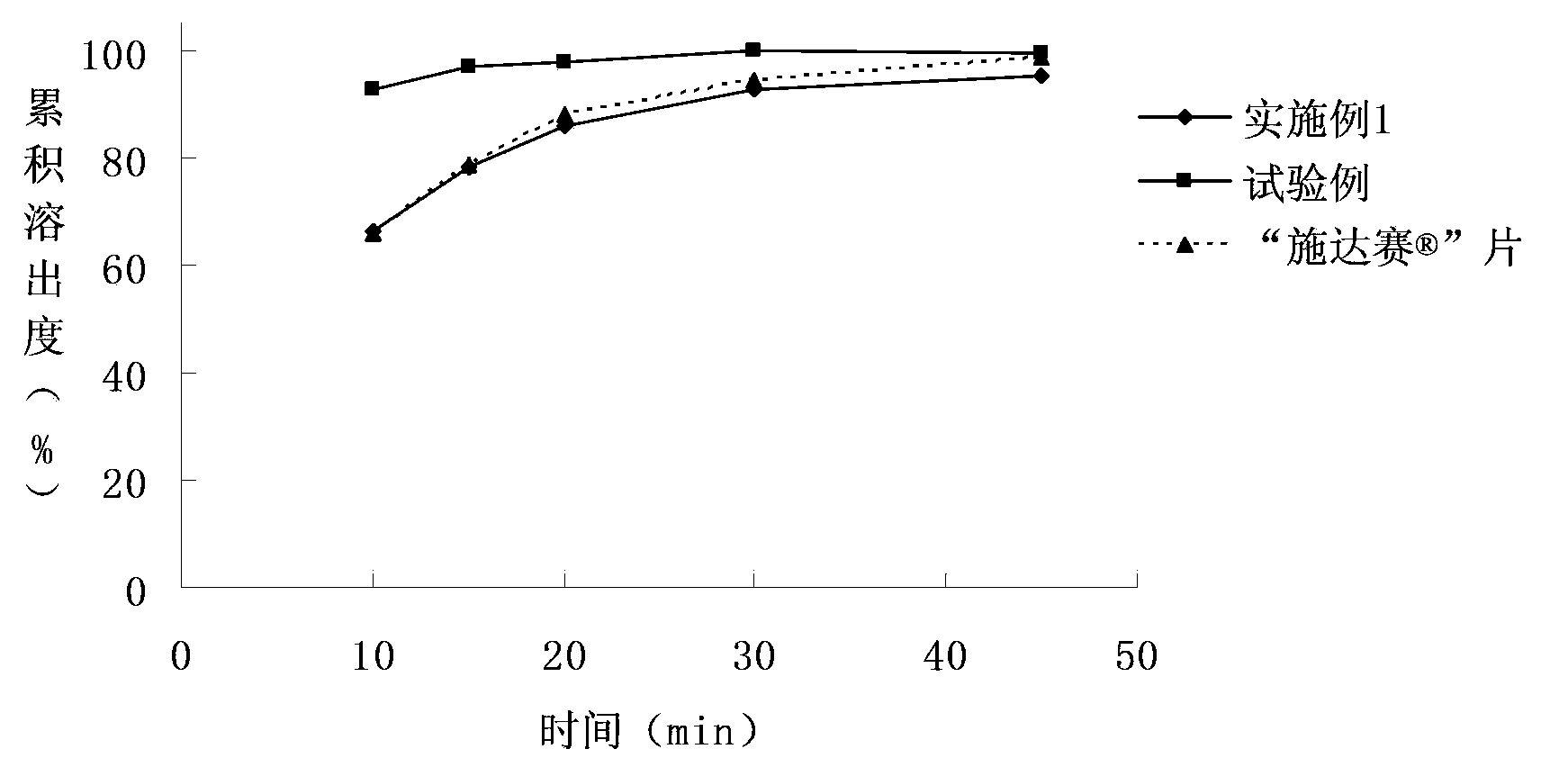
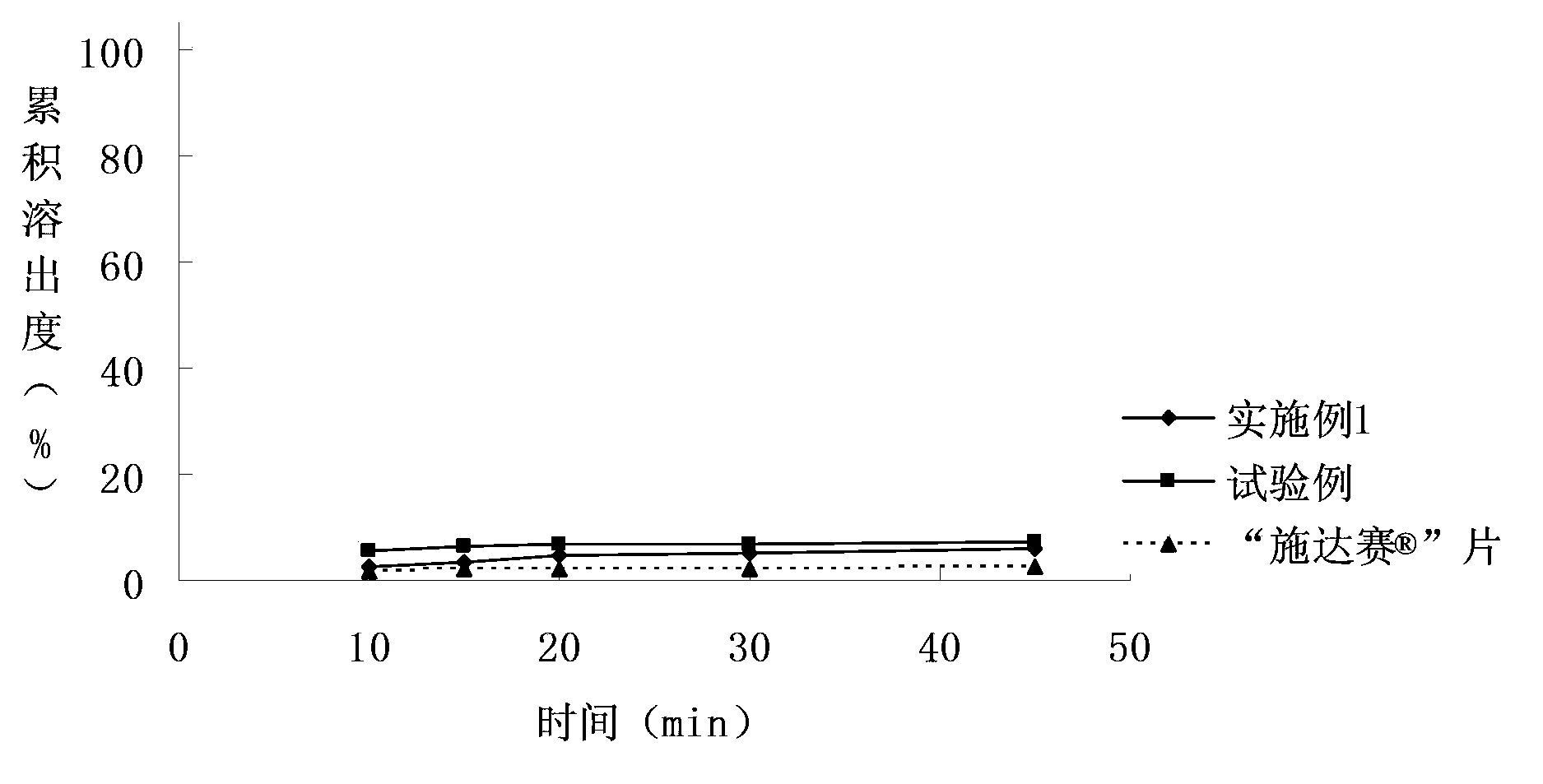
[0053] The dasatinib tablet of embodiment 1 and test example and " Shi Da The comparison chart of the dissolution curves of "tablets in different dissolution media" Figure 1~3 shown.
Embodiment 2
[0054] Example 2. Dasatinib Tablets Prescription Composition: The specification is 70 mg (calculated as Dasatinib), and the prescription quantity is 1000 tablets.
[0055]
[0056] Preparation method: mix the prescription amount of dasatinib anhydrous with lactose, microcrystalline cellulose, methylcellulose and hydroxypropyl cellulose evenly, add 90g of purified water to wet granulate, dry, and then add the prescription amount The crospovidone and magnesium stearate were mixed evenly and compressed into tablets.
[0057] The dasatinib tablet of embodiment 2 and " Shi Da The comparison chart of the dissolution curves of "tablets in different dissolution media" Figure 4~6 shown.
Embodiment 3
[0058] Example 3. Dasatinib Tablet Prescription Composition: The specification is 70 mg (calculated as Dasatinib), and the prescription quantity is 1000 tablets.
[0059]
[0060] Preparation method: mix the prescribed amount of dasatinib anhydrous with lactose, microcrystalline cellulose, low-substituted hydroxypropyl cellulose, hydroxypropyl methyl cellulose and povidone, and add 90g of purified water to wet granulate , dried, then add the prescribed amount of magnesium stearate, mix well, and compress into tablets.
[0061] The dasatinib tablet of embodiment 3 and " Shi Da The comparison chart of the dissolution curves of "tablets in different dissolution media" Figure 7~9 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com