New crystal of Dasatinib monohydrate and preparation method thereof
A technology of monohydrate and dasatinib, applied in the preparation method and pharmaceutical composition thereof, in the field of crystal form III of dasatinib monohydrate, which can solve the problems of difficult crystal quality and difficult to handle organic waste liquid, and achieve Easy operation, high crystal purity and good process repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
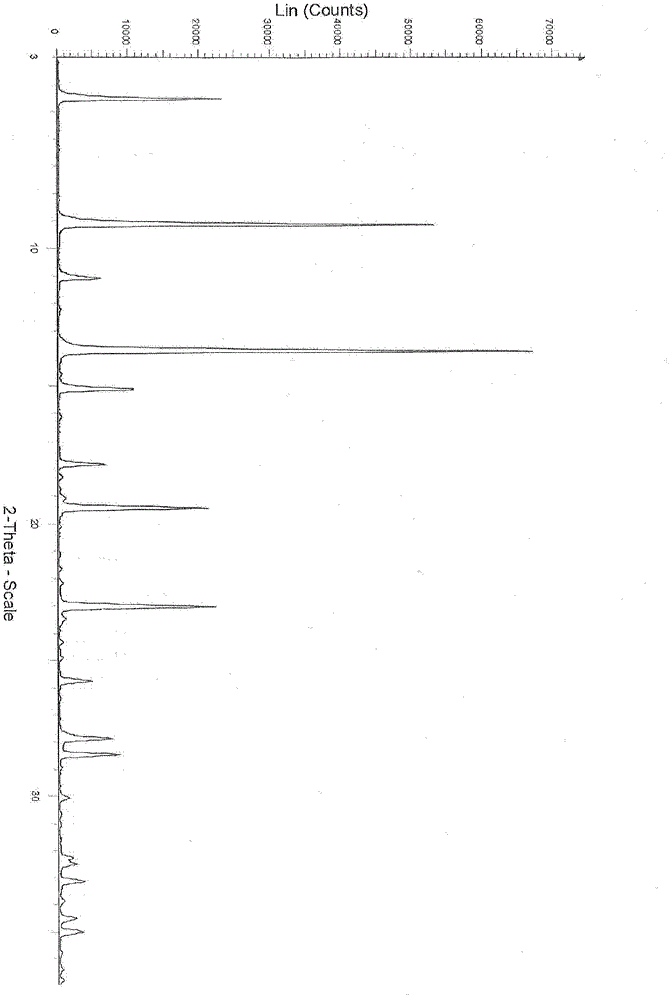
[0056] Preparation of dasatinib monohydrate crystal form III
[0057] Take 28g of dasatinib and add it to 2000ml of methanol, stir and heat until dissolved, filter it once while it is hot, add 1400ml of water to the filtrate while stirring, stop stirring, let the temperature drop naturally, slowly precipitate fine crystals, and then add it under stirring at room temperature after 3 hours 600ml of water, stop stirring after adding, leave to crystallize. The precipitated white crystals were filtered by suction, washed with a small amount of methanol, dried under vacuum at 60°C, and assisted by phosphorus pentoxide to obtain 23 g of the product as a white crystalline powder.
Embodiment 2
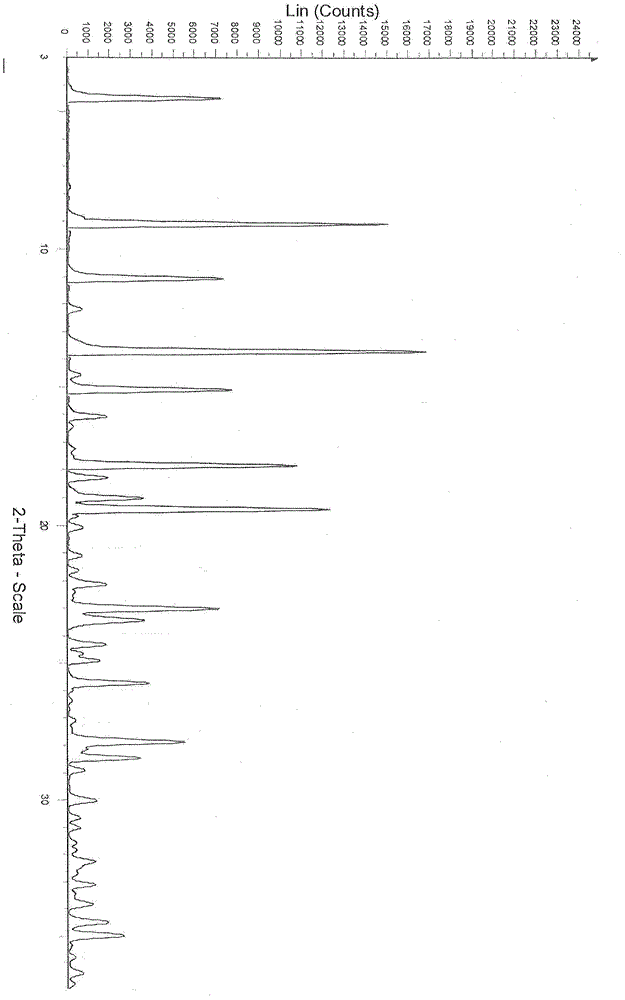
[0059] Preparation of dasatinib monohydrate crystal form III
[0060] Take 10g of Dasatinib and add it to 1000ml of acetone, stir and heat to reflux to dissolve, filter once while it is hot, add 500ml of water to the filtrate while stirring, stop stirring, cool down naturally, slowly precipitate fine crystals, and stir again at room temperature after 4 hours. Add 500ml of water, stop stirring after adding, and leave to crystallize. The precipitated white crystals were filtered by suction, washed with a small amount, and dried under vacuum at 60° C., assisted by phosphorus pentoxide to obtain 8.4 g of the product as a white crystalline powder.
Embodiment 3
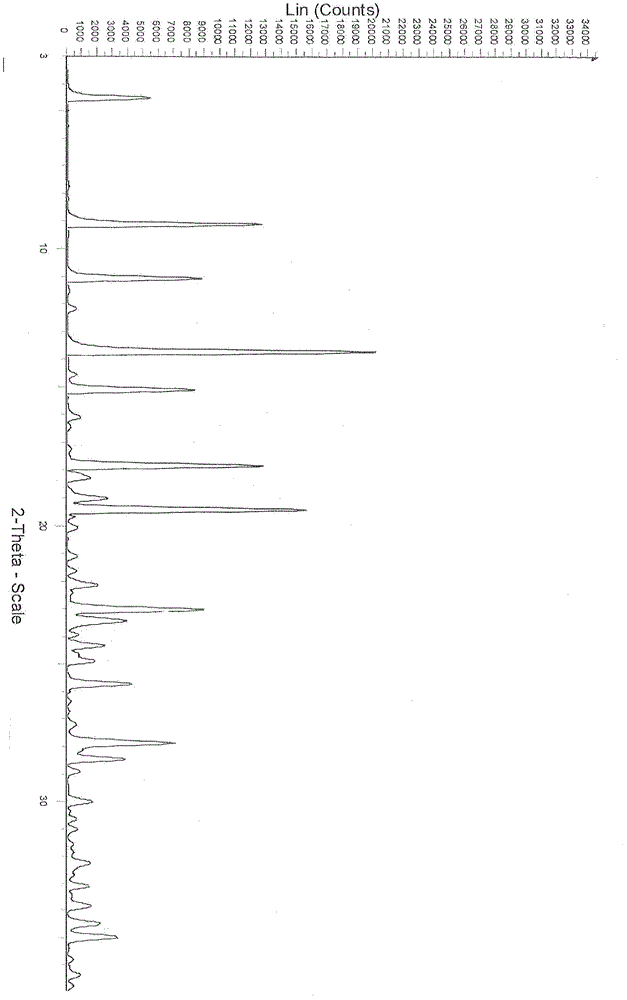
[0062] Preparation of dasatinib monohydrate crystal form III
[0063] Take 10g of Dasatinib and add it to 50ml of dimethylformamide, heat it to 70°C under stirring to dissolve, filter once while it is hot, add 75ml of water to the filtrate while stirring, stop stirring, and cool down naturally until fine crystals are slowly precipitated, at room temperature Add 30ml of water under stirring, stop stirring after adding, and leave to crystallize. The precipitated white crystals were filtered by suction, washed with a small amount, and dried under vacuum at 60° C., assisted by phosphorus pentoxide to obtain 7.3 g of the product as a white crystalline powder.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com