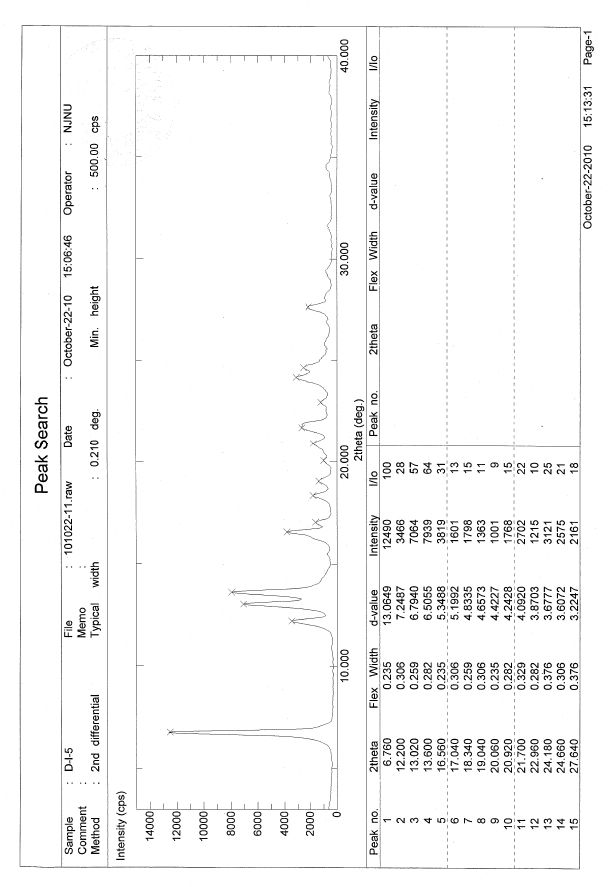
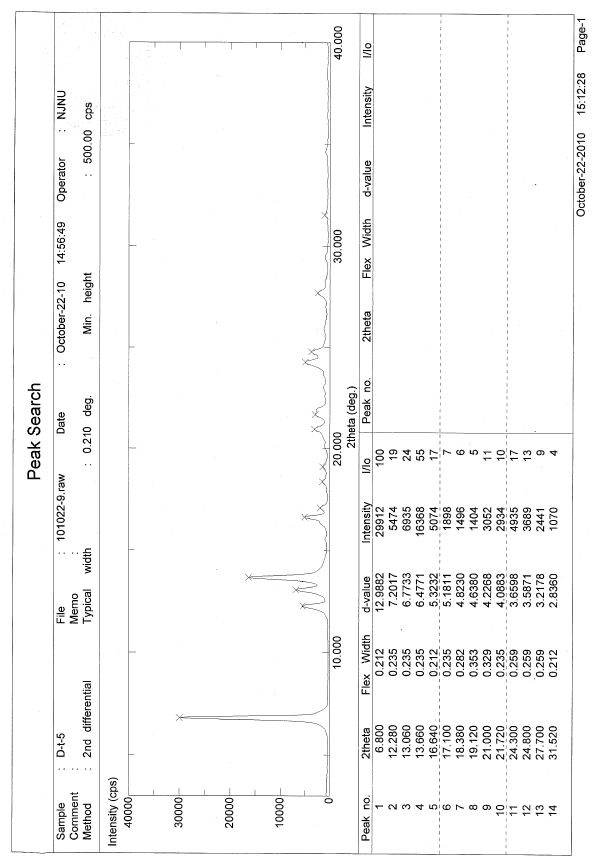
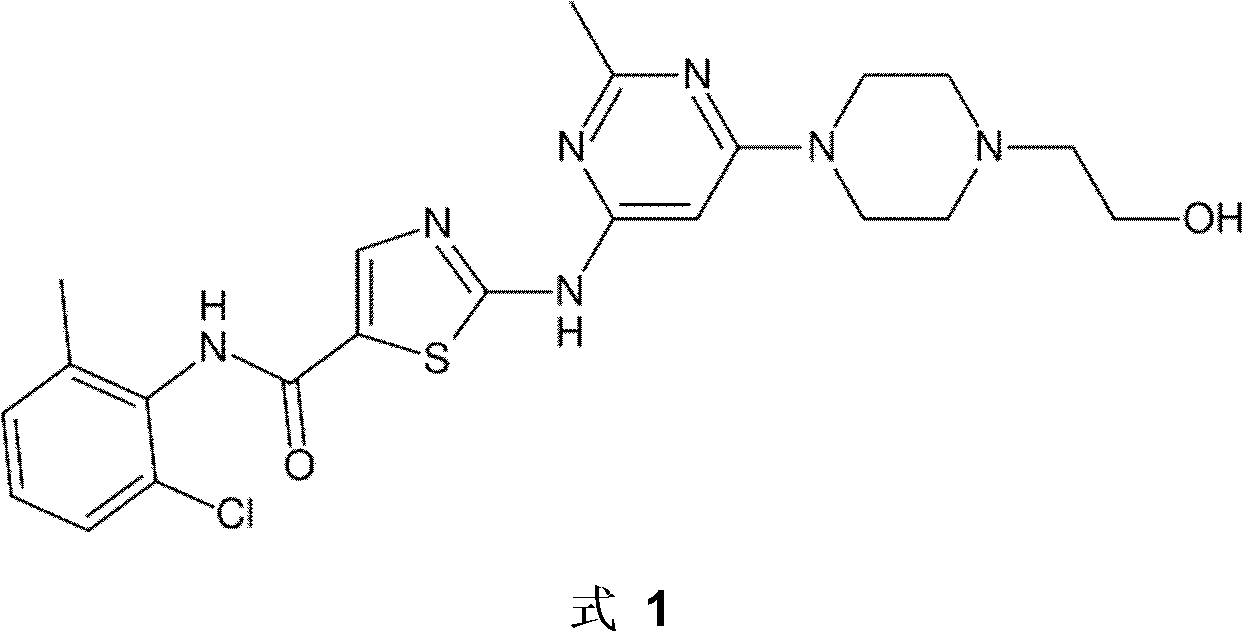
A new preparation method for Dasatinib N-6 crystal form
A crystal form and crystal technology, applied in the field of preparation of dasatinib N-6 crystal form, can solve the problems of cumbersome operation and inability to realize industrialized production, and achieve the effects of strong operability, low cost and strong controllability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Embodiment 1: Preparation of Dasatinib N-6 crystal form
[0018] Weigh Dasatinib (487mg, 1mmol), then add it to the reaction flask, add 13mL methanol, control the temperature at 70°C, stir magnetically, slowly drop to 20°C after dissolving, filter, and wash with cold methanol (3×2mL) , dried under vacuum at 30°C to obtain 478 mg of Dasatinib N-6 crystal form, with a yield of 98%.
Embodiment 2
[0019] Embodiment 2: Preparation of Dasatinib N-6 crystal form
[0020] Weigh Dasatinib (487mg, 1mmol), then add it to the reaction flask, add 20mL methanol, control the temperature at 70°C, stir magnetically, slowly drop to 15°C after dissolving, filter, and wash with cold methanol (3×2mL) , dried under vacuum at 30°C to obtain 428 mg of Dasatinib N-6 crystal form, with a yield of 88%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com